Current options for third-line therapy and beyond in metastatic colorectal cancer
Authors:
Dr. med. Guido Pesola
Medical Oncology Department
Oncology Institute of Southern Switzerland (IOSI)
Ente Ospedaliero Cantonale (EOC), Bellinzona
E-Mail: guido.pesola@eoc.ch
PD Dr. med. Sara De Dosso
Medical Oncology Department
Oncology Institute of Southern Switzerland (IOSI)
Ente Ospedaliero Cantonale (EOC), Bellinzona
& Faculty of Biomedical Sciences
USI, Lugano, Switzerland
Vielen Dank für Ihr Interesse!
Einige Inhalte sind aufgrund rechtlicher Bestimmungen nur für registrierte Nutzer bzw. medizinisches Fachpersonal zugänglich.
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
Treating metastatic colorectal cancer (mCRC) in third- and fourth-line settings remains a complex challenge. Advancements in molecular profiling have enabled tailored treatments based on tumor characteristics. Also, third- and fourth-line treatment options have consistently demonstrated survival benefits across diverse patient groups. Choosing the best treatment option and sequence of care requires careful patient selection and an understanding of the treatment options based on an analysis of the scientific evidence available to date. This article explores how molecular insights and innovative strategies are shaping the treatment landscape for advanced mCRC.
Keypoints
-
Only 25–30% of patients reach third-line therapy, with even fewer proceeding to fourth line.
-
At advanced stages, treatment focuses on prolonging survival, maintaining quality of life, and managing symptoms.
-
Trials such as SUNLIGHT and FRESCO-2 provide evidence for improved progression-free and overall survival in heavily pretreated patients without actionable mutations.
-
Targeted therapies, such as KRAS G12C inhibitors, EGFR inhibitors rechallenge, and HER2-targeted regimens, are emerging as effective options for specific molecular subgroups.
Metastatic colorectal cancer (mCRC) is a major global health concern, consistently ranking as one of the leading causes of cancer-related mortality. Although systemic therapies have significantly enhanced outcomes in earlier treatment lines, disease progression to third- and fourth-line settings continues to pose substantial challenges, with limited therapeutic options and poorer prognoses.
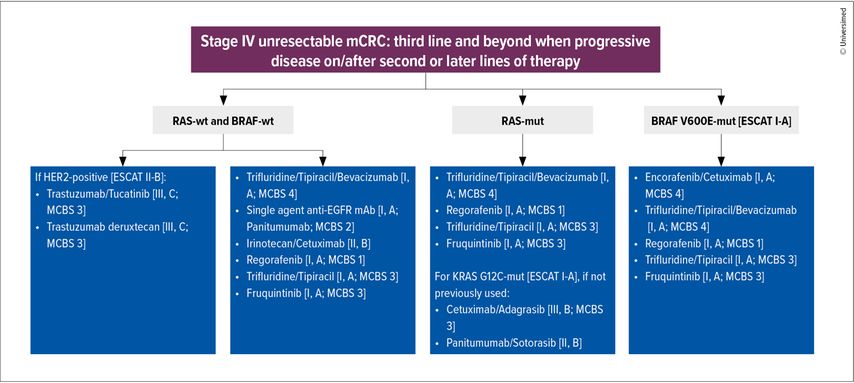
The advent of novel therapeutics, drug combinations, and molecular profiling have significantly refined treatment paradigms, allowing for the identification of actionable mutations and opening pathways for targeted therapies with improved precision and efficacy. As a result of these advances, an update to the ESMO living guidelines was released in September 2024, expanding the therapeutic arsenal available to oncologists while leaving many options open for clinical decision-making (Fig. 1).1 This article delves into the characteristics of patients receiving third- and fourth-line treatments, and the latest evidence supporting contemporary clinical practices.
Fig. 1: As treatment options proliferate, new possibilities open up for patients, but uncertainties remain for clinicians (modified after ESMO Metastatic Colorectal Cancer Living Guideline. Version: v1.2 - September 2024)1
Treatment goals
At advanced stages of metastatic colorectal cancer, only a fraction of patients proceed to later lines of treatment, with approximately 25–30% reaching third-line and 10–15% progressing to fourth-line therapies.2 Challenges are significant, as more than 85% of patients at the third-line stage exhibit no actionable mutations, and salvage or retreatment chemotherapy demonstrates limited or no activity.3
Until a few years ago, median overall survival (mOS) from the start of third-line treatment was approximately 6.4 months, with median progression-free survival (mPFS) of 2.6 months in third-line and 2.4 months in fourth-line settings. These data underscore the importance of balancing efficacy with toxicity to meet the critical goals of sustaining quality of life (QoL) and performance status (PS).4
Third-line therapy
Historically, regorafenib and trifluridine/tipiracil were the standard options for third-line treatment in mCRC. These therapies provided similar efficacy, though their distinct toxicity profiles often influenced treatment choice. Regorafenib was associated with fatigue, hepatotoxicity, and hand-foot syndrome, whereas trifluridine/tipiracil was predominantly linked to hematological toxicities.5,6
The optimal sequencing of these agents remained unclear; however at ESMO GI 2024, the SOREGATT trial demonstrated that initiating treatment with trifluridine/tipiracil followed by regorafenib resulted in better tolerability and fewer toxicities.7
The landscape of third-line therapy has since evolved with the results of the SUNLIGHT trial, which established the combination of trifluridine/tipiracil and bevacizumab as the new standard of care. This regimen significantly improved PFS (5.6 months vs. 2.4 months) and overall survival (10.8 months vs. 7.5 months) compared to trifluridine/tipiracil alone.8
Fourth-line therapy
In the third- and fourth-line setting, fruquintinib (a VEGFR 1–3 inhibitor) has emerged as a promising option. Data from the FRESCO-2 trial demonstrated that fruquintinib improved PFS to 3.7 months and overall survival (OS) to 7.4 months, compared to 1.8 and 4.8 months, respectively, in patients receiving placebo. The safety profile of fruquintinib was favorable, with a low incidence of grade 3 adverse events.9
Targetable molecular subgroups
Metastatic CRC encompasses a diverse array of molecular subtypes, each requiring tailored therapeutic approaches.
Although standard-of-care (SoC) lines were studied for all-comers, patients with tumor-related symptoms and a high disease burden may benefit more significantly from targeted therapies. Studies report notable response rates, ranging from 19.5% to 45.3% depending on the specific mutation.10
KRAS G12C inhibitors, such as sotorasib, adagrasib and divarasib, have demonstrated promising outcomes when combined with anti-EGFR agents, with studies highlighting meaningful response rates and survival improvements.11–13 It remains unclear, though, whether these patients should receive SoC or targeted therapies in chemo-refractory settings. According to Van de Haar’s study, patients with the KRAS G12C mutation did not benefit from trifluridine/tipiracil alone, while a recent post-hoc analysis of the SUNLIGHT trial with bevacizumab reported no prognostic impact of KRAS G12 on the OS.14,15
For patients with RAS/BRAF wild-type and HER2 amplification, HER2-targeted regimens, including trastuzumab/tucatinib and trastuzumab deruxtecan, have demonstrated significant efficacy in refractory cases and are the preferred option in the third-line setting.16,17
Rechallenge strategies with EGFR inhibitors can be an option, based on prior treatment history and residual sensitivity to EGFR blockade in tumor clones. The rechallenge of EGFR inhibitors in patients hyperselected with ctDNA, as shown in the CHRONOS, CITRIC and VELO trials, has proven to be a viable strategy, improving disease control and PFS.18–20
Conclusion
Effective management of metastatic colorectal cancer requires a balance between evidence-based standard therapies and emerging targeted approaches. Molecular profiling and tailored strategies are crucial for improving outcomes in heavily pretreated patients. Future efforts should prioritize integrating novel agents and refining treatment sequencing to enhance survival and quality of life.
Literatur:
1 Cervantes A et al.: Ann Oncol 2024; 35(2): 241-3 2 Abrams TA et al.: J Natl Cancer Inst 2014; 106: djt371 3 Nielsen DL et al.: Cancer Treat Rev 2014; 40(5): 500-7 4 Koopman M et al.: Ann Oncol 2023; 34(Suppl): S434-5 5 Grothey A et al.: Lancet 2013; 381(9863): 303-12 6 Mayer RJ et al.: N Engl J Med 2015; 372(20): 1909-19 7 Cremolini C et al.: J Clin Oncol 2023; 41(suppl): Abstr. #3501 8 Prager GW et al.: N Engl J Med 2023; 388(18): 1657-67 9 Dasari A et al.: Lancet 2023; 402(10395): 41-53 10 Stucchi E et al.: Expert Opin Pharmacother 2024; 25(4): 371-82 11 Fakih M: N Engl J Med 2023; 389: 2125-39 12 Yaeger R: Cancer Discov 2024; 14 (6): 982-93 13 Desai J et al.: Nat Med 2024; 30(1): 271-8 14 Van de Haar J et al.: Nat Med 2023; 29(3): 605-14 15 Tabernero J et al.: ESMO Open 2024; 9(3): 102945 16 Strickler J et al.: ASCO 2024, Abstr. #3509 17 Yoshino T: Nat Commun 2023; 14(1): 3332 18Sartore-Bianchi A et al.: Nat Med 2022; 28(8): 1612-8 19 Santos C et al.: Ann Oncol 2024; 35(suppl_ 2): S428-81 20 Napolitano S et al.: JAMA Oncol 2023; 9(7): 966-70
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...