Multiple Myeloma: What’s new?
Author:
Dr. med. Rouven Müller
Oberarzt meV
Klinik für Medizinische Onkologie und Hämatologie USZ
Facharzt Hämatologie (FMH) und Allgemeine Innere Medizin (FMH)
FA Hämatologie/Onkologie (D)
Universitätsspital Zürich
E-Mail: rouven.mueller@usz.ch
Vielen Dank für Ihr Interesse!
Einige Inhalte sind aufgrund rechtlicher Bestimmungen nur für registrierte Nutzer bzw. medizinisches Fachpersonal zugänglich.
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
Last year’s ASH annual meeting again provided a wealth of new data in the field of myeloma, from promising results regarding the 4-drug combinations in induction and consolidation therapy to immunotherapeutic approaches. Further, first data of the nationwide Iceland screening study iStopMM with 148708 Icelanders were presented.
The importance of early disease detection: iStopMM
One highlight of last year’s ASH meeting represented by several abstracts and oral presentations was the nationwide screening study from Iceland, the iStopMM study.1,2 The aim of this trial was to study screening of monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM) systematically in order to benefit from early detection that potentially could lead to better disease control or even cure.
The rationale for this study is based on previous findings of the same group showing that myeloma patients that got their diagnosis after previously having been followed for a diagnosis of MGUS have a better outcome than patients that got their diagnosis of myeloma without knowledge and follow-up for a MGUS before.3 Similar data was published for cohorts of MGUS patients that progressed to myeloma that showed less fractures, kidney disease, hypercalcemia and superior survival than cohorts that got the diagnosis of myeloma without previous follow-up for a known MGUS.4
Participants of the iStopMM trial were screened via serum protein electrophoresis, immunofixation and free light-chain (FLC) assay and were either diagnosed as having MGUS, advanced disease which is smouldering myeloma (SMM) or even myeloma (if M protein was ≥30g/l or FLC ratio ≥100) or normal screening. Patients with MGUS were either randomized to having no further workup (arm 1), were followed according to IMWG guidelines (arm 2) or a more intensified follow-up with bone marrow examination and imaging.
Between November 2016 and October 2018 148708 Icelanders age >40 were eligible of whom 80759 (54.3%) gave their consent and 75422 (93.4%) were sampled. 3725 patients (4.9%) were diagnosed MGUS with age dependance (2.3% (40–59y), 6.2% (60–79y) and 12.9% (80–103y) and higher prevalence in males than in females (5.9 vs. 4.1%, p<0.0001). Based on the randomized follow-up strategy after 3 years, in the three treatment arms n=9, 92 and 133 lymphoproliferative disorders were diagnosed for arm 1–3 respectively, meaning a statistically significant increase in diagnosis of lymphoproliferative disorders in the arms with intensified follow-up (arm 2 and 3). The difference was also significant when looking at subgroups for the entities smouldering Waldenström’s macroglobulinemia, SMM and MM. These results demonstrate that screening identifies a significant higher number of patients with smouldering disease at risk or established malignancy that requires treatment offering the opportunity of an early intervention.
Abstract #151 from the same group focused separately on the prevalence of smouldering MM, a precursor condition of MM where early treatment might be beneficial as emerging data are indicating. So far a screening strategy for smouldering myeloma is not standard and the diagnosis is made incidentally when workup for MGUS is done. Screening of 75422 Icelanders revealed 3725 patients with detected paraprotein. Bone marrow sampling was done in 1503 patients of these and 180 patients got the diagnosis of smouldering myeloma after additional imaging and exclusion of SLiM-CRAB criteria.
To estimate the prevalence of smouldering myeloma in the general population the group looked at the MGUS population described above that was randomized to arm 3, where all the patients (n=1279) received a bone marrow biopsy as part of the intensified follow-up strategy. The prevalence of SMM in this population was 10.8% and the prevalence of SMM in the total population >40 years was estimated to be 0.53%. The identification of smouldering myeloma cases by screening with high prevalence is especially important given the fact that current risk stratification models consider roughly one third of these patients to be of intermediate or high risk, a patient population that might benefit from early intervention at this early disease stage in the future.
Daratumumab to RVd in induction and consolidation: GRIFFIN
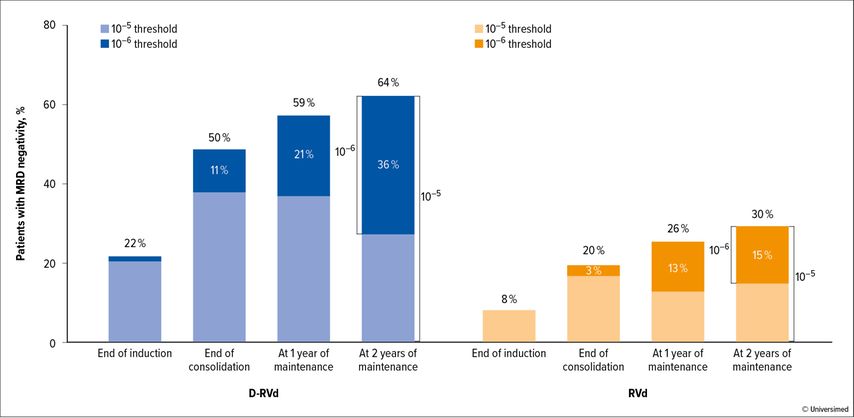
Beside early stages of plasma-cell dyscrasias there were some interesting updates on therapy optimization trials, investigating 4-drug combinations in the newly diagnosed transplant-elegible setting. The phase II GRIFFIN trial exploring the combination of daratumumab, bortezomib, lenalidomide and dexamethasone (Dara-RVd) as induction, post-ASCT (autologous stem cell transplantation) consolidation followed by Dara-Len vs. Len maintenance presented updated results after 24 months of maintenance.5 The addition of Dara to RVd in induction and consolidation after 24 months of maintenance showed – again – as in previous updates of that trial improved rates for stringent complete remission (sCR) and MRD(minimal residual disease)negativity at the 10-5 and 10-6 level (Fig. 1). Although not powered for that, a trend to improved progression-free survival (PFS) was seen.
Fig. 1: GRIFFIN trial. MRDneg rate during induction and consolidation therapy with Dara-VRd or VRd, followed by maintenance therapy with daratumumab-lenalidomide or lenalidomide only (25 cycles) (modified from Laubach et al.)5
Isatuximab + Rvd: GMMGH-HD7
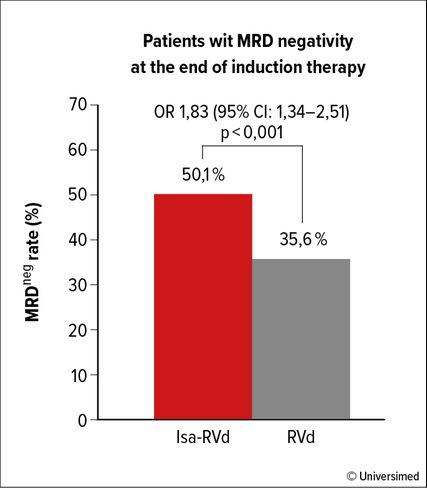
Fig. 2: MRDneg rate after 3 cycles of VRd induction therapy with/without isatuximab (modified from Goldschmidt et al.)6
With a similar design but using the CD38 targeting antibody isatuximab the German phase III GMMGH-HD7 trial showed in an early interim analysis that the addition of isatuximab to standard RVd in induction improves MRD negativity rates at the 10-5 level using next generation flow cytometry at a very early timepoint after three 42-day cycles of RVd +/- isatuximab induction (Isa-RVd 50.1% vs. RVd 35.6%, p<0.001; Fig. 2).6 Further follow-up of this trial will inform about the potential benefit of adding isatuximab to lenalidomide vs. lenalidomide alone in maintenance therapy randomized in a second step after high-dose melphalan and autologous stem-cell transplantation.
MRD-tailored approach: 4-drug combination Dara-KRd in MASTER
Another trial investigating quadruple induction and consolidation in newly diagnosed MM patients eligible for transplantation was presented by US investigators that conducted the MASTER trial.7 In this trial patients are treated with the combination of daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd). Anticipating a very high activity of this regimen, there was a planned enrichment in the recruitment of patients with high-risk cytogenetic abnormalities [gain 1q, t(4;14), t(14;16), t(14;20) or del(17p)].
Recognizing the robust predictive value of MRD-negativity for overall survival (OS) and PFS as well as the burden and impact on quality of life of an indefinite maintenance therapy, the investigators used MRD testing via next-generation sequencing (NGS) to tailor the intensity of consolidation therapy and to cessate treatment in patients with confirmed MRD negativity. Patients received 4 cycles of Dara-KRd as induction, high dose therapy and ASCT and according to their MRD status 0, 4 or 8 cycles of Dara-KRd. Patients received therapy until two consecutive MRD testings were <10-5, after that patients entered treatment free observation and surveillance of MRD 6 months after treatment cessation and yearly thereafter. Patients completing 8 cycles of Dara-KRd consolidation without achieving MRD negative status entered maintenance phase with lenalidomide.
Of the 123 recruited patients 43% had no high-risk cytogenetic aberration, 37% had one and 20% had ≥2 high-risk aberrations. Median age was 60years, in 95.9% of patients NGS-MRD testing could be established and median follow up was 25.1 months. 80% of the patients achieved MRD negativity and 66% achieved MRD negativity at a level of less than 10-6. Of the patients with 0, 1, and ≥2 high-risk cytogenetic abnormalities MRD negativity was 78, 82 and 79%, the 2y-PFS 91, 97 and 58% and OS 96, 100 and 75% respectively. The cumulative incidence of MRD reappearance or progression according to IMWG criteria within 12 months after treatment cessation was 4%, 0% and 27% for 0, 1, ≥2 HR abnormalities respectively.
Summarizing the first analysis of this interesting MRD tailored approach the 4-drug combination of daratumumab-KRd was able to induce a very high rate of MRD negativity in newly diagnosed MM (NDMM) especially taking into consideration the enrichment of high-risk patients in this trial. Nearly all of the patients with 0 or 1 high-risk features achieving MRD negativity remained in remission despite treatment cessation while the ultra-high-risk group with ≥2 HR cytogenetic abnormalities warrants for more intensive consolidation strategies showed by significantly reduced 2y-PFS and OS.
MS-based methodology in Myeloma XI
The previous abstract shows an example how MRD analytics might guide future treatment selection and cessation. The methodology of MRD is well established but the frequency of assessment is somehow limited by the invasive nature of repetitive bone marrow assays and results might be influenced by the heterogenous involvement of bone marrow biopsy sites or in the presence of extramedullary disease.
Addressing this, a group from Birmingham reported a mass spectrometry (MS)-based methodology in sensitively measuring free-light-chains (FLCs) in peripheral blood in patients with transplant-eligible newly diagnosed MM.8 293 patients treated in the Myeloma XI trial (induction with KRCd, ASCT, randomization lenalidomide maintenance vs. no maintenance) were included in the analysis. Available samples were collected from post-induction, day 100 after ASCT and post-maintenance randomization. The serum samples were immunoprecipitated with antisera specific for FLC kappa or lambda and coupled to magnetic particles followed by analysis on MALDI-TOF (matrix-assisted laser desorption ionization-time of flight) MS.
At all time points assessed the authors could show that MS positivity was significantly associated with shorter PFS. Interestingly, 47 patients could be monitored with bone marrow MRD testing (8-color flow panel with sensitivity of 4x10-5) in parallel at the post-maintenance randomization. Within these 35 (74.5%) were MRD negative and 12 (25.5%) were MRD positive. 6 of the 35 (17.1%) MRD negative patients still tested positive for residual FLC in MS and after a median follow-up of 42.2 months FLC-MS positivity in MRD negative patients was associated with a statistically significant shorter PFS (33.3% progression in MRD neg. FLC MS positive vs. 6.9% in MRD neg. FLC MS negative patients, p=0.001).
This study exemplarily shows that FLC MS could contribute to sensitive disease monitoring and may add prognostic information. If this holds true if the most sensitive MRD monitoring techniques up to the 10-6 level are applied will be clarified in future studies, nevertheless the ease of acquiring peripheral blood serum samples will support the further clinical development of this approach.
Immunotherapeutic approaches
FcRH5-CD3 bispecific Cevostamab
Another huge topic of the annual meeting 2021 were again updates or first reports of early-phase clinical trials of immunotherapeutic approaches in the relapsed and refractory setting namely bispecific antibodies and CAR(chimeric antigen receptor)-T-cell therapies.
An interesting target on myeloma cells is the Fc receptor-homolog 5 (FcRH5) which is expressed exclusively in the B-cell lineage and predominantly on myeloma cells.9 Cevostamab, a bispecific antibody targeting FcRH5 on myeloma cells and the epsilon domain of CD3 T cells, directing these to killing of myeloma cells, already showed promising activity during the early Phase I testing phase.10
Dr. Trudel from Toronto presented updated results of the phase I dose-finding phase in a heavily pretreated (median 6 prior therapies) patient population.11 The cevostamab bispecific in the dose-finding phase was able to induce a target-dose dependent overall response between 29 and 54.8% with an early median time to first response of 1 month and cytokine-release-syndrome mitigation by using a step-up dosing approach justifying further investigation and expansion of the clinical evaluation.
GPRC5D-CD3-bispecific Talquetamab
A second promising target that could be exploited immunotherapeutically represents the GPRC5D (G protein coupled receptor class c group 5 member D) which is highly expressed on MM plasma cells. Talquetamab, a bispecific antibody recruiting CD3 T-cells to GPRC5 leading to T-cell activation and subsequent lysis of target myeloma cells is currently investigated in the Phase I trial MonumenTAL-1, (NCT03399799). At ASH congress 2021, updated results of patients treated with the two recommended phase II doses (RP2D) of 405ug/kg sc every week or 800ug/k sc every 2 weeks were presented.12
Again, a heavily pretreated patient population with a median of 5 to 6 prior therapies, approx. 22% with prior BCMA(B-cell maturation antigen)-targeted therapy and of note approximately 3/4 triple-class refractory (≥1 proteasome inhibitor [PI], ≥1 immunomodulatory imide drugs [IMiD], ≥1 anti-CD38 monoclonal antibody [mAb]) and 1/4 penta-class refractory (≥2 PI, ≥2 IMiD, ≥1 anti-CD38 mAb) patients were included. In both dosings an overall response rate of 60–70% overall and especially in the triple- and penta-class refractory could be achieved, with more than 50% achieving a very good partial response (VGPR) or better overall response. Responses occurred early after approx. 1 month and were durable and deepened over time pending longer follow-up. Beside the classical side effects of CRS, talquetamab shows some skin-related and nail adverse events reasoned by the expression of GPRC5 in the affected organs. Both was manageable and did not affect further investigation.
New follow-up data of CARTITUDE-1
From the cellular side of immunotherapy a promising update on the CARTITUDE-1 trial was reported after a longer follow-up of median two years.13 CARTITUDE-1 is a phase Ib/II trial that investigates ciltacabtagene autoleucel (cilta-cel), a CAR-T construct directed against BCMA in relapsed-refractory MM. The cilta-cel design contains two binding domains for BCMA that confer improved avidity to the target cell. Efficacy reported at previous meetings at follow up of 12.4 months already showed a high overall response rate of 97% with stringent CR of 67% and a manageable safety profile.
The 2 year median follow up update could show now an impressive 97.9% overall response rate with an 67% sCR at one year improving to 82.5% sCR rate in current update. 24-months PFS was approx. 71% in patients achieving a CR and 60% in all patients. Again MRDnegativity at the level of 10-5 sustained for 6 or 12 months was predictive for an improved PFS and OS. No new safety signals were reported.
In a nutshell
To summarize, last year’s ASH annual meeting again provided a wealth of new data in the myeloma field. Further follow-up of Iceland’s iStopMM will provide exiting data based on this population-wide screening initiative for plasma-cell dyscrasias. In the newly diagnosed setting of MMtransplant eligible the 4-drug combinations will define future standard of care.
Since MRD is now a validated surrogate marker for PFS and OS and therefore earlier readout of clinical trials is possible, we expect accelerated knowledge gain in clinical trials and aim for MRD informed treatment decisions to titrate therapy intensity individually. Further study of new MRD techniques like mass spectrometry of easy accessible serum-free light chains from peripheral blood will inform if MRD status can be refined and shifted to higher sensitivity levels.
Finally the identification of new myeloma specific antigens and exploitation of new and established antigens by immunotherapy either by bispecifics, antibody-drug conjugates or CAR-T-cells shows already very promising results in the heavily pretreated relapsed refractory setting with high tumor burden and is expected to be even more beneficial when implemented in earlier clinical settings of lower tumour burden or even MRD to eradicate remaining myeloma cells.
Literature:
1 Kristinsson SY et al.: ASH 2021; Abstr. #156 2 Thorsteinsdottir S et al.: ASH 2021; Abstr. #151 3 Sigurdardottir EE et al.: JAMA Oncology 2015; 1(2): 168-74 4 Go RS et al.: Clin Lymphoma Myeloma Leuk 2015; 15(3): 177-186.e4 5 Laubach J et al.: ASH 2021; Abstr. #79 6 Goldschmidt H et al.: ASH 2021; Abstr. #463 7 Costa LJ et al.: ASH 2021; Abstr. #481 8 Giles HV et al.: ASH 2021; Abstr. #820 9 Li J et al.: Cancer Cell 2017; 13; 31(3): 383-95 10 Cohen AD et al.: ASH 2020; Abstr. #292 11 Trudel S et al.: ASH 2021; Abstr. #157 12 Krishnan A et al.: ASH 2021; Abstr. #158 13 Martin T et al.: ASH 2021; Abstr. #549
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...