Is there still a role for allo-HSCT in CML?
Authors:
Dr. med. Frederica Giannotti
Cheffe de Clinique
Prof. Dr. med. Yves Chalandon
Médecin Chef de Service
Service d‘Hématologie
Hôpitaux Universitaires de Genève
E-Mail: yves.chalandon@hcuge.ch
Tyrosine kinase inhibitor (TKI) therapy in CML has dramatically reduced the number of patients requiring allogeneic hematopoietic stem cell transplantation (allo-HSCT). This procedure remains a very effective therapeutic option in chronic phase CML resistant or intolerant to TKIs, and in accelerated phase CML. Advances in transplant procedure and donor availability have increased transplant feasibility and improved outcome. The ability to identify patients whose outcome if treated with TKIs will be poor and continuous improvements in transplant outcome will likely increase the role of allo-HSCT in CML.
Keypoints
-
With the advent of targeted therapy, allo-HSCT use has decreased; however it is of importance to monitor closely patients who are under TKI and avoid that they progress to advanced phase (AP or BC) as the outcome after transplant is better for CP1 as compared to all other conditions.
-
Allo-HSCT should be considered for patient with BC after their return to CP, for those progressing from CP to AP and for the one in CP after failure of third-line therapy or with T315I mutation.
-
In the future, it will be important to be able to anticipate which CML patients will not respond well to TKI and will therefore be candidate for allo-HSCT.
Chronic myeloid leukemia: anoverview
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder (MPN) of the hematopoietic stem cell (HSC) characterized by the presence of the 22q- or «Philadelphia» (Ph) chromosome, formed by the reciprocal translocation, t(9;22) (q34;q11), encoding the BCR-ABL1 fusion gene. It accounts for 15% to 20% of adult leukaemias, being the most common of the MPN.1 The worldwide annual incidence of CML is 0.4–2 cases/100000 persons with inter-country variations; it increases with age and has a slight male predominance. The median age at presentation is between 57 and 60 years in Europe.2 The majority of patients (90–95%) is diagnosed in chronic phase (CP), a relatively indolent condition easily controlled with treatment. The natural history continues with a bi- or triphasic stage, becoming more aggressive through accelerated phase (AP) and then blast crisis (BC) or directly from CP to BC.3,4 Several multivariate-derived prognostic models and staging systems have been proposed to help define individual prognosis and allow assigning patients to different strategies of therapy based on risks. The most commonly used are the Sokal and the Hasford scores.5,6 The ELN have attempted to improve upon these scores using large cohorts of patients treated with tyrosine kinase inhibitors (TKIs) from diagnosis, proposing the EUTOS score7 to predict complete cytogenetic response (CCyR) at 18 months. EUTOS has not proved to be a consistent predictor of overall survival (OS) or progression-free survival (PFS), suggesting that CML itself is now a rare cause of death and patients are more likely to die for other medical conditions. The latest EUTOS long-term survival score (ELTS) has been developed to try to predict death from disease.8 Although these scoring systems are useful, particularly for choosing first-line therapy, the most important prognostic indicators for CML remain phase of disease at diagnosis and the speed and depth of response to TKI therapy.
Treatment of chronic myeloid leukemia
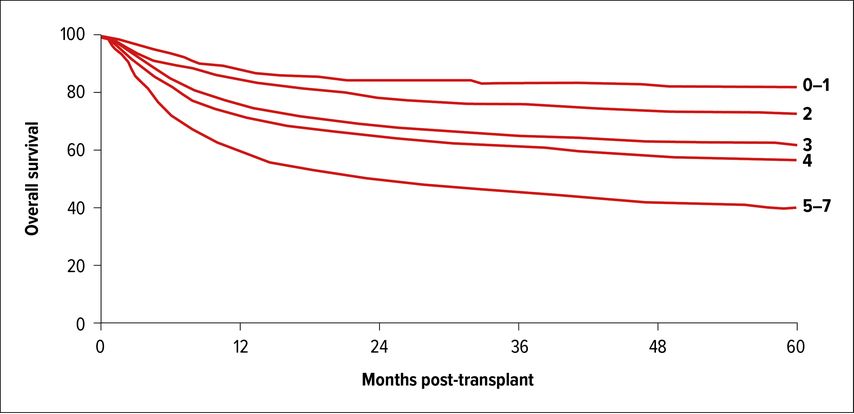
Before the development of TKIs, CML patients who did not undergo allo-HSCT had a median survival of 3–5 years in the busulfan (BU) and hydroxycarbamide era and 6–8 years in the interferon-alpha (IFN-α) era. Only 30–60% of patients survived beyond 10 years. Early CML therapy included radiotherapy, at the beginning of the 20th century, and later oral chemotherapy, with haematological responses in 50–80% of thecases. In the early 80s IFN-α showed a survival advantage inducing complete hematologic responses of 40–80%, with cytogenetic response of 15–58% and CCyR of 5–25%, and was recommended as first-line therapy until 2001. Later association of IFN-α with cytarabine produced higher cytogenetic response rate at 12 months (61%), and significantly better survival (5-year rate of 70%).9 Nevertheless, these approaches could control the signs and symptoms of CML but could not prevent transformation into a rapidly fatal chemoresistant blastic disease. The first curative treatment that eradicated the Ph+ cells was bone marrow transplant, initially described in syngeneic twins and soon followed by procedures involving HLA-matched siblings and later unrelated donors (URD).10–12 Autologous HSCT started about at the same time, in the late 70s, aiming to set up the clock to early phase in transformed disease and in patients refractory to IFN-α.13 Following the introduction of TKIs, the number of auto-HSCT has decreased rapidly, and it is currently not a recommended strategy in CML. The benefit of allo-HSCT is to provide definitive cure, but the clear disadvantage is its association with considerable morbidity and mortality.14 Outcome can be improved by better selection of those most likely to benefit. Gratwohl et al. developed the EBMT risk score for patients with CML based on five variables: donor type, disease phase, recipient age, donor/recipient gender combination, and interval from diagnosis to transplant, which together result in a score of 0–7.15 The EBMT score was highly predictive for survival and transplant related mortality (TRM) in a big validating cohort of 5018 patients transplanted from 1989–1997.16 During this period, allo-HSCT was the treatment of choice for all patients. Since 2000 allo-HSCT has been replaced by TKIs as frontline therapy, and hence the reasons for patients undergoing transplant are not always clear from registry data. Interpretation of more recent results should be made with some caution. In CML it is worth focusing specifically on the impact of disease phase, because one of the few problems of TKIs therapy is that within the cohort of patients receiving transplants, the proportion transplanted in or after BC has increased over time. In fact, allografts were initially restricted to AP CML, and improvements in survival came only when transplant was performed in CP.17 The effect of disease phase on the outcome of transplantation has not changed over the years. Nevertheless, an analysis recently repeated by the CMWP of the EBMT for 3497 patients transplanted from 2007 to 2017 confirmed improved outcome of 5-year OS across all-risk scores by 11–26% (Fig.1).18
Tyrosine kinase inhibor therapy in CML
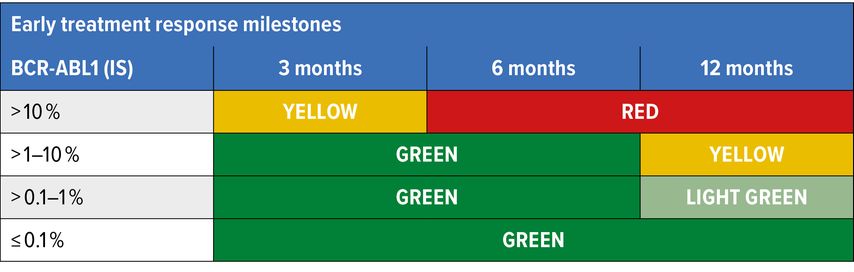
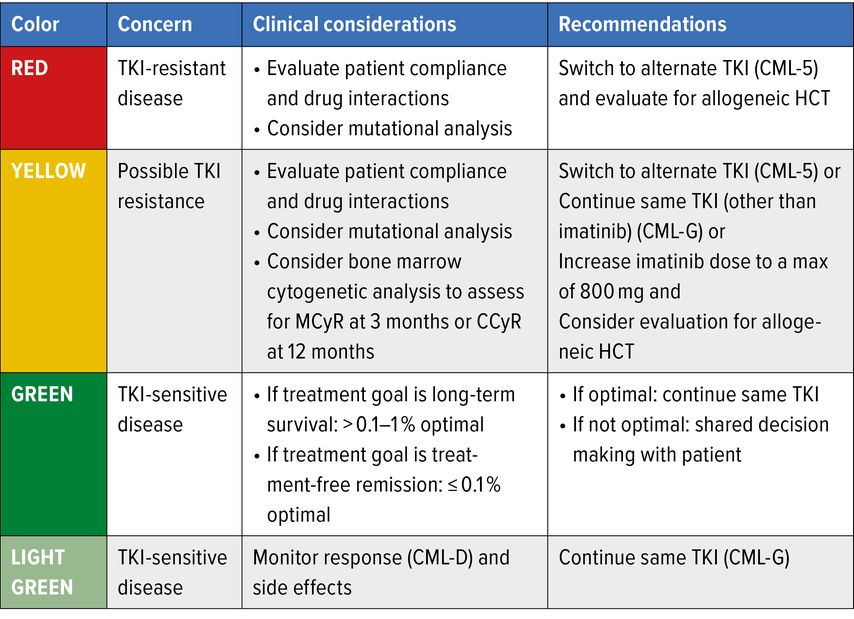
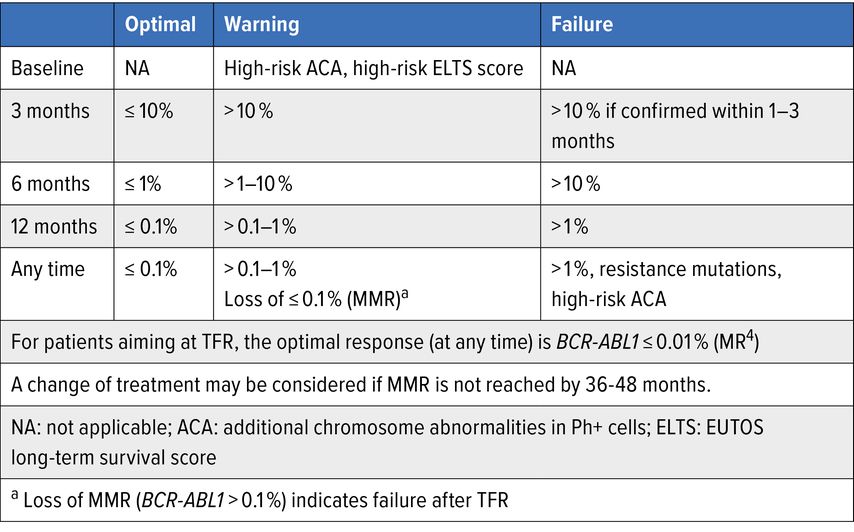
Development of TKIs has radically changed the standard therapeutic approach for all phases of CML. Nowadays, in western countries, the life expectancy of a newly diagnosed Ph+ CML in CP is very close to that of age-matched individuals in the general population.19,20 The phase III International Randomized Study comparing imatinib to the combination of IFNα and cytarabine (IRIS study), which leaded to regulatory approval of imatinib in 2001, has been recently updated showing a dramatic change in the prognosis of CML in TKIs era. With a median follow-up of 10.9 years, the OS at 10 years of patients treated with imatinib was 83.3%, and 97.8% when the analysis was limited to CML-related deaths, in patients achieving major molecular response (MMR: <0.1% BCR-ABL1 IS). Moreover, there were low rates of progression to AP or BP in the imatinib group (6.9%, 0%–0.9% per year from year 4 to 8).21–23 Since 2018, 4 other TKIs have been approved for 1st and 2nd line treatment of CML, all representing excellent choices, with different toxicity profiles. The efficacy of 2nd and 3d generation TKIs (2GTKI and 3GTKI) has led to their use as 1st line therapy, and recently completed phase III studies suggest that approximately 80% of patients will achieve CCyR within the 1st year, compared to 65% on imatinib, with a more rapid achievement of MMR.24,25 Based on these results, dasatinib, nilotinib and bosutinib have been licensed for use in newly diagnosed patients. The 3GTKI ponatinib, the only one that is effective against T315I mutation, was then approved to treat T315I-mutated CML.26 Other benefits have been reported for 1st-line use of 2GTKI as compared to imatinib, including the development of fewer mutations conferring TKI resistance and decreased rates of progression to AP and BP.1 Still, imatinib remains the most cost-effective choice and also the TKI with the longest safety track record. Nevertheless, 2- and 3GTKI can be preferred in specific situations. The response to TKIs has a significant impact on prognosis and response criteria are well defined. Guidelines for milestones for response and recommendations for monitoring and intervention in TKI therapy have been created by ELN and NCCN and are based upon IS molecular response (Fig. 2a,b).27,28
Fig. 1: OS curves supplied by Mrs. Linda Koster for the EBMT CMWP and based on 3497 CML patients transplanted from 2007 to 2017, according to EBMT risk score
Importantly, the main goals in CML treatment are:
to reach response milestones, in order to ensure normal life span
to optimize quality of life while taking daily medication
to minimize longer-term potentially irreversible toxicities
to achieve such deep and durable molecular responses that TKI discontinuation can be considered, resulting in «treatment free remission» (TFR).
Consequently, even if achievement of response milestones represents the main factor guiding therapeutic decisions, both specific TKIs toxicities and patient’s comorbidities, together with their personal therapeutic goals, have to be taken in account for an optimal CML treatment decision–making, as well as adherence assessment and mutational screening results.29, 30
Fig. 2: Guidelines regarding milestones for response and recommendations for monitoring and intervention in TKI therapy
a) NCCN Guideline Version 2.2021
Allo-HSCT in CML nowadays
Despite the dramatic changes in CML treatment in the last 2 decades, allo-HSCT still has a place in patients cure, ensuring long-term PFS.31 This is particularly true as the leukemic quiescent stem cells are not dependent on BCR-ABL signaling for survival, and are not targeted by TKIs, leading to a proportion of patients who will relapse or will have resistant disease despite TKI therapy. A potent graft-versus-leukemia (GVL) effect was early demonstrated in CML by registry studies that showed a decreased risk of relapse in patients developing chronic graft-versus-host-disease (GvHD) and increased relapse rates in those receiving in vivo T-cell depletion (TCD).32 The importance of the GVL effect was subsequently supported by the observation that donor lymphocyte infusion (DLI) could restore durable molecular remissions in CML patients relapsing after allo-HSCT.33 These findings led to the development of reduced intensity conditioning regimens (RIC), which extended transplant to older and unfit patients with TKI resistant or intolerant CML in whom allograft might previously be associated with unacceptable toxicity.34
Allo-HSCT, once the treatment of choice for CP CML patients, has been relegated to 2nd-, 3rd- or 4th-line treatment after the development of TKIs. With extended follow-up, it appears that 60% of patients can achieve excellent long-term disease control on imatinib, and a small proportion may even be able to stop treatment without experiencing disease recurrence.22 Approximately half of this group will achieve or regain remission on one of the 2GTKI or 3GTKI.35–37 In addition, 70–80% of patients receiving 2GTKI as 1st-line therapy will achieve durable responses, of these 20–30% will require ponatinib, since patients who are resistant to 2GTKI have a low probability of achieving a durable response to an alternative 2GTKI.26,38,39 Allo-HSCT is the therapy of choice for CP CML who failed to respond, develop TKI resistant mutations, and lose an established response and/or are intolerant to the drug. Retrospective registry and single-center studies report 3-year survival rates ranging from 70% to 90% in patients in CP transplanted with a matched sibling donor after a myeloablative conditioning (MAC) regimen.40,41 The time to proceed to transplant remains controversial, particularly for the substantial number of patients being started on 2GTKI as 1st-line therapy, who, in case of resistance, progression, or relapse, may be rescued with either another 2GTKI or 3GTKI, and for whom the question to proceed to transplant immediately or wait for another progression and 3rd-line therapy rescue before allo-HSCT is a matter of debate. This is less true for those who are failing 3rd-line therapy or have T315I mutation, for whom allo-HSCT is recommended.42 Failure to respond to ponatinib after 3 months therapy indicates a patient at high risk of progression, and early transplant is indicated.43,44 A number of national and international study groups are now reporting that long-term response to imatinib and 2GTKI can be predicted by the rate of fall of BCR-ABL transcript levels (as measured by RQ-PCR at 3 and 6 months).45 It is therefore possible to identify patients destined for transplant within the 1st year of diagnosis while still in CP and return to a more measured approach to it. Moreover, as more potent TKIs move to 1st-line therapy, patients destined to respond poorly to these drugs are identified earlier, and transplant will return to use as an earlier-line strategy. A small number of patients develop serious cytopenias after exposure to TKIs, with insufficient residual haematopoiesis to repopulate the bone marrow. For these, allo-HSCT is the only effective therapy.
Allo-HSCT remains the therapy of choice for AP CML. For de novo AP patients, search for a donor and referral to a transplant centre should be considered at diagnosis and TKIs serve as a bridge to transplant. Outcome in AP CML treated with TKIs alone compares unfavourably with those achieved in CP (20–40% achieve CCyR and responses are often not durable). Nevertheless, for AP CML without additional cytogenetic aberrations nor elevated blast percentage, HSCT may be deferred depending on response to 1st-line TKI therapy.46,47 For patients progressing from CP to AP during treatment, transplant should be considered immediately after obtaining a new response to TKI as their outcome is not good without. Five-years PFS rates of 50%–80% have been reported in patients with AP CML allografted using a sibling or URD.
For patients presenting in, or progressing to BP, the long-term outcome with any of the currently available TKI is poor,48,49 although a subset of patients with blasts percentage <30% may have outcomes similar to AP patients. Allo-HSCT should be offered after initial control of the disease, since inducing a second CP in BP CML yields outcomes comparable to AP CML.50 The addition of a TKI to chemotherapy-based AML or ALL regimens improves the chance of achieving CP.51,52 Thereafter, patient should proceed to transplant without delay since PFS in BP is low and time to allo-HSCT plays a crucial role.53 Long-term survival rates for patients who achieve a second CP range from 30% to 40% after a transplant that used a MAC regimen. Transplantation in BP is not recommended.
Recently the EBMT-CMWP analysed data of patients transplanted for CML in the 3GTKI era showing that the number of TKIs given prior to allo-HSCT seems not to impact on outcome. However, the stage of the disease, as also reported by the CIBMTR, as also the performance status did have an important impact.54,55 It is therefore very important to try to keep patients in 1st CP and avoid progression, even for those rescued to ≥CP2 after having progressed to advanced phase, as the results after allograft are worse in this group.
Source of HSC
About 2/3 of the transplants done nowadays for CML use peripheral blood stem cells (PBSC) as source of HSC, similarly to what is seen in other haematological malignancies.12 There is no difference in general outcome depending on the HSC source, although bone marrow may have a decrease incidence of chronic GvHD and its severity.56 The source of HSC is therefore left open, but PBSC may potentially be preferred to decrease the risk of graft failure and relapse in more advanced disease, particularly with the use of RIC.
Over time, HSCT from all type of donors were performed in CML. Some reports suggest that relapse rate with matchedURDs could be lower than after matched-related transplantation and minor antigen disparity could enhance GVL effect.57
Conditioning regimen and GvHD prophylaxis
For CML patients, the best conditioning regimen as well as the best GvHD prophylaxis remain to be determined. Regarding MAC, CY combined either with BU or TBI is still the one that has shown the best overall long-term survival.58 RIC has been introduced later and has dramatically increased the number of patients who could potentially benefit from it, resulting in encouraging results over time.59,60 Still, it did not show improved outcome over MAC, particularly in relation with a higher incidence of relapse.61,62 Therefore, for elderly or patients with comorbidities, RIC (FLU with BU or MEL) will be the choice, and for the others, particularly with advanced phases, MAC should be proposed for a better control of the disease. For GvHD prophylaxis, CsA with short course MTX remains the standard.58 In order to reduce incidence and severity of GvHD, TCD was introduced in the 80s, showing increased relapse rates,32 which led to many groups abandoning TCD in sibling andalso in URD allografts for CML. Others continued to use TCD and have reported good outcomes in sibling transplants, particularly following the introduction of DLI. In a small series of 23 CML patients with a median age of 36 years transplanted with sibling donors and MAC between 1998 and 2016 at the University Hospital of Geneva using partialTCD with alemtuzumab, the 15-year OS and LFS were 95% using the strategy of escalating doses DLI for early molecular relapses, with a low incidence of acute and chronic GvHD (Chalandon, unpublished data).
Strategies after allo-HSCT
Thirty to 70% of patients transplanted for CML will relapse and several factors were identified to determine risk of disease recurrence as AP CML, use of RIC and TCD.50 After allo-HSCT, rising or persistently high levels of BCR-ABL1 mRNA can be detected prior to cytogenetic or haematological relapse. Low or falling BCR-ABL1 transcript levels are associated with continuous remission, while high or rising transcript levels predict relapse. Therefore monitoring BCR-ABL1 post-allo-HSCT for CML is of outmost importance to identify patients at higher risk of relapse and permit early therapeutic interventions, even in the long term, due to relapses occurring >15 years post-HSCT.63,64 Many CML patients will remain RQ-PCRpositive during the first 3 months after allo-HSCT, especially in the RIC era or using TCD. In patients who are at least 4 months post-allo-HSCT, a working definition of molecular relapse is one of the following:
-
BCR-ABL/ABL1 ratio higher than 0.02% in 3 samples a minimum of 4 weeks apart
-
Clearly rising BCR-ABL/ABL1 ratio in 3 samples a minimum of 4 weeks apart with the last 2 higher than 0.02%
BCR-ABL/ABL1 ratio higher than 0.05% in 2 samples a minimum of 4 weeks apart65
DLI remains the most effective salvage therapy in patients relapsing after allo-HSCT, allowing remission re-induction in 60–90% of patients with CML transplanted and relapsing in CP. The use of escalating doses in case of persistent disease reduces the risk of GvHD.66–68 An EBMT study showed 69% 5-year survival in 328 patients who received DLI for relapsed CML. DLI-related mortality was 11%, and disease-related mortality was 20%. Some form of GvHD was observed in 38% of patients. Risk factors for developing GvHD after DLI were T-cell dose at first DLI, time interval from transplant to DLI and donor type. In multivariate analysis, GvHD after DLI was associated with an increased risk of death.69
With the advent of TKI, CML post-transplant interventions are more complex but give more opportunities to rescue patients. TKI therapy is often effective in the setting of post-transplant relapse and can be used when GvHD is present and DLI is not an option.70 It is possible to combine DLI and TKI for relapsing patients; however, the best order (TKI first, DLI first, or both combined) has not yet been defined.71 The EBMT-CMWP reported 431 patients with CML relapses post-allo-HSCT who received TKI either alone (55%) or in combination with DLI (14.5% before, 4.4% at the same time, and 26% after TKI). Only 42% of the patient obtained either a complete molecular (17.7%), cytogenetic (4.4%), or haematological (20.2%) remission with a 5-year OS of 60% and of 47% for RFS. This rather low response rate may be in relation with the fact that 235 patients were transplanted for advanced phases (AP, BC or > CP1).72 Outcomes for patients with AP CML at relapse are not as good. A recent study of 14 AP patients reported CCyR rates of 71% and undetectable BCR-ABL1 transcripts in 57%, either with imatinib or dasatinib treatment alone or in combination with DLI and achievement of undetectable transcripts was very strongly associated with OS.73Therefore, in high risk patients maintenance with TKI is often recommended for at least 1 year after transplant.74,75
Literature:
1 Jabbour E et al.: myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol 2020; 95(6): 691-709 2 Hoglund M et al.: Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol 2015; 94 Suppl 2: 241-7 3 Chereda B, Melo J.V: Natural course and biology of CML. Ann Hematol 2015; 94 Suppl 2: 107-21 4 Bonifacio M et al.: Management of chronic myeloid leukemia in advanced phase. Front Oncol 2019; 9: 1132 5 Sokal J.E et al.: Prognostic discrimination in „good-risk“ chronic granulocytic leukemia. Blood 1984; 63(4): 789-99 6 Hasford J et al.: A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998; 90(11): 850-8 7 Hasford J et al.: Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 2011; 118(3): 686-92 8 Pfirrmann M et al.: The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia. Leukemia 2020; 34(8): 2138-49 9 Garcia-Manero G et al.: Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer 2003; 98(3): 437-57 10 Champlin R et al.: Allogeneic bone marrow transplantation for chronic myelogenous leukemia in chronic or accelerated phase. Blood 1982; 60(4): 1038-41 11 Arora M et al.: HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. JClin Oncol 2009; 27(10): 1644-52 12 Holtick U et al.: Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev, 2014(4): CD010189 13 Olavarria E: Autologous stem cell transplantation in chronic myeloid leukemia. Semin Hematol 2007; 44(4): 252-8 14 Craddock CF: We do still transplant CML, don‘t we? Hematology Am Soc Hematol Educ Program 2018; 2018(1): 177-84 15 Gratwohl A et al.: Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998; 352(9134): 1087-92 16 Passweg J.R et al.: Validation and extension of the EBMT Risk Score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol 2004; 125(5): 613-20 17 Speck B et al.: Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet 1984; 1(8378): 665-8 18 Data provided by Mrs L. Koster on behalf of the EBMT CMWP 19 Bower H et al.: Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol 2016; 34(24): 2851-7 20 Sasaki K et al.: Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol 2015; 2(5): 186-93 21 O‘Brien S.G et al.: Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348(11): 994-1004 22 Hochhaus A et al.: Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 2017; 376(10): 917-27 23 Hehlmann R et al.: Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017; 31(11): 2398-406 24 Cortes J.E et al.: Final 5-year study results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J Clin Oncol 2016; 34(20): 2333-40 25 Fachi M.M et al.: Comparative efficacy and safety of tyrosine kinase inhibitors for chronic myeloid leukaemia: A systematic review and network meta-analysis. Eur J Cancer 2018; 104: 9-20 26 Cortes J.E et al.: Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 2018; 132(4): 393-404 27 Hochhaus A et al.: European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020; 34(4): 966-984 28 NCCN Guidelines Version 2.2021 29 Cortes J: How to manage CML patients with comorbidities. Hematology Am Soc Hematol Educ Program 2020; 2020(1): 237-42 30 Braun TP et al.: Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell 2020; 37(4): 530-42 31 Lubking A et al.: Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry. Bone Marrow Transplant 2019; 54(11): 1764-74 32 Apperley JF et al.: Bone marrow transplantation for patients with chronic myeloid leukaemia: T-cell depletion with Campath-1 reduces the incidence of graft-versus-host disease but may increase the risk of leukaemic relapse. Bone Marrow Transplant 1986; 1(1): 53-66 33 Mackinnon S et al.: Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood 1995; 86(4): 1261-8 34 McSweeney PA et al.: Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97(11): 3390-400 35 Kantarjian H.M et al.: Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011; 117(4): 1141-5 36 Kantarjian H et al.: Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood 2007; 109(12): 5143-50 37 Kantarjian HM et al.: Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood 2014; 123(9): 1309-18 38 Milojkovic D et al.: Responses to second-line tyrosine kinase inhibitors are durable: an intention-to-treat analysis in chronic myeloid leukemia patients. Blood 2012; 119(8): 1838-43 39 Milojkovic D et al.: Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica 2010 95(2): 224-31 40 Innes AJ et al.: Allogeneic transplantation for CML in the TKI era: striking the right balance. Nat Rev Clin Oncol 2016; 13(2): 79-91 41 Saussele S et al.: Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood 2010; 115(10): 1880-5 42 Xu LP et al.: Allogeneic stem cell transplantation for patients with T315I BCR-ABL Mutated chronic myeloid leukemia. Biol Blood Marrow Transplant, 2016. 22(6): p. 1080-6 43 Nicolini FE et al.: Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer 2017; 123(15): 2875-80 44 Boddu P et al.: Life after ponatinib failure: outcomes of chronic and accelerated phase CML patients who discontinued ponatinib in the salvage setting. Leuk Lymphoma 2018; 59(6): 1312-22 45 Branford S et al.: Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol 2012; 30(35): 4323-9 46 Moynihan JB, Ennis S: Acetazolamide-insensitive carbonic anhydrase activities in liver and tonic skeletal muscle of adult male rats with streptozotocin-induced diabetes mellitus. Biochem J 1990; 272(2): 553-6 47 Silver RT et al.: Sustained durability of responses and improved progression-free and overall survival with imatinib treatment for accelerated phase and blast crisis chronic myeloid leukemia: long-term follow-up of the STI571 0102 and 0109 trials. Haematologica 2009; 94(5): 743-4 48 Hehlmann R et al.: Management of CML-blast crisis. Best Pract Res Clin Haematol 2016; 29(3): 295-307 49 Soderlund S et al.: Advanced phase chronic myeloid leukaemia (CML) in the tyrosine kinase inhibitor era - a report from the Swedish CML register. Eur J Haematol 2017; 98(1): 57-66 50 Jain P et al.: Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017; 123(22): 4391-4402 51 Strati P et al.: HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer 2014; 120(3): 373-80 52 Milojkovic D et al.: Efficacy of combining dasatinib and FLAG-IDA for patients with chronic myeloid leukemia in blastic transformation. Haematologica 2012; 97(3): 473-4 53 Gratwohl A et al.: Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia 2016; 30(3): 562-9 54 Chalandon Y et al.: Allogeneic stem cell transplantation in patients with CML-CP in the era of third generation tyrosine kinase inhibitors: a study by the CMWP of the EBMT. EBMT annual meeting, Lisbon, Portugal 2018 55 CIBMTR annual report 2017 56 Anasetti C et al.: Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012; 367(16): 1487-96 57 McGlave PB et al.: Unrelated donor marrow transplantation for chronic myelogenous leukemia: 9 years‘ experience of the national marrow donor program. Blood 2000; 95(7): 2219-25 58 Copelan EA: Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354(17): 1813-26 59 Warlick E et al.: Reduced intensity conditioning is superior to nonmyeloablative conditioning for older chronic myelogenous leukemia patients undergoing hematopoietic cell transplant during the tyrosine kinase inhibitor era. Blood 2012; 119(17): 4083-90 60 Crawley C et al.: Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood 2005; 106(9): 2969-76 61 Kebriaei P et al.: Long-term follow-up of allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning for patients with chronic myeloid leukemia. Blood 2007; 110(9): 3456-62 62 Chhabra S et al.: Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Adv 2018; 2(21): 2922-2936 63 Mo XD et al.: Preventing relapse after haematopoietic stem cell transplantation for acute leukaemia: the role of post-transplantation minimal residual disease (MRD) monitoring and MRD-directed intervention. Br J Haematol 2017; 179(2): 184-97 64 Goldman J.M et al.: Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol 2010; 28(11): 1888-95 65 Kaeda J et al.: Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: an attempt to define patients who may not require further therapy. Blood 2006; 107(10): 4171-6 66 Kolb HJ et al.: Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86(5): 2041-50 67 Dazzi F et al.: Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood 2000; 95(1): 67-71 68 Dazzi F et al.: Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000; 96(8): 2712-6 69 Chalandon Y et al.: Outcome of patients developing GVHD after DLI given to treat CML relapse: astudy by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant 2010; 45(3): 558-64 70 Egan DN et al.: Patients with Philadelphia-positive leukemia with BCR-ABL kinase mutations before allogeneic transplantation predominantly relapse with the same mutation. Biol Blood Marrow Transplant 2015; 21(1): 184-9 71 Olavarria E et al.: Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia 2003; 17(9): 1707-12 72 Chalandon Y et al.: Use of first or second generation TKI for CML after allogeneic hematopoietic stem cell transplantation: astudy by the CMWP of the EBMT. EBMT annual meeting, Marseille, France, 2017 73 Savani BN et al.: Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant 2005; 36(11): 1009-15 74 DeFilipp Z et al.: Maintenance tyrosine kinase inhibitors following allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia: ACenter for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant 2020; 26(3): 472-9 75 DeFilipp Z et al.: Does post-transplant maintenance therapy with tyrosine kinase inhibitors improve outcomes of patients with high-risk philadelphia chromosome-positive leukemia? Clin Lymphoma Myeloma Leuk 2016; 16(8): 466-71
Das könnte Sie auch interessieren:
Erhaltungstherapie mit Atezolizumab nach adjuvanter Chemotherapie
Die zusätzliche adjuvante Gabe von Atezolizumab nach kompletter Resektion und adjuvanter Chemotherapie führte in der IMpower010-Studie zu einem signifikant verlängerten krankheitsfreien ...
Highlights zu Lymphomen
Assoc.Prof. Dr. Thomas Melchardt, PhD zu diesjährigen Highlights des ASCO und EHA im Bereich der Lymphome, darunter die Ergebnisse der Studien SHINE und ECHELON-1
Aktualisierte Ergebnisse für Blinatumomab bei neu diagnostizierten Patienten
Die Ergebnisse der D-ALBA-Studie bestätigen die Chemotherapie-freie Induktions- und Konsolidierungsstrategie bei erwachsenen Patienten mit Ph+ ALL. Mit einer 3-jährigen ...