Patient-specific lateralization, glenoid offset, implications and operative strategy
Ensemble Hospitalier de la Côte,<br>Clinique de Morges, Switzerland<br>E-Mail: stefan.bauer@ehc.vd.ch
Centre Orthopédique Santy,<br>Hôpital privé Jean-Mermoz, Lyon, France
Roth/McFarlane Hand & Upper Limb Centre,<br>London (Ontario), Canada
Although it seems that some degree of lateralization is beneficial for reverse shoulder arthroplasty (RSA), the ideal amount of global lateralization and the ideal contribution from the glenoid side and humeral stem remain unknown. Preoperative 3D planning with calculation of virtual rigid body ROM (range of motion) is important to appreciate the need for increased glenoid offset for impingement-free ROM to avoid notching especially in younger patients. Patient age and life expectancy, bone quality, patient height, body mass index as well as intra-operative gap tension assessment need to be considered for patient-specific RSAlateralization.
Keypoints
-
There has been a shift from the Grammont design to lateralized RSA increasing stability.
-
Notching is associated with poor longterm RSA outcomes.
-
Decreased NSA with respect to classical Grammont design is key to limit notching.
-
Increased glenoid offset lengthens the scapula neck and decreases notching effectively.
-
Preoperative 3D planning with analysis of ROM is invaluable.
-
Lateralization should be adapted to individual patient factors.
-
Operative gap assessment is recommended.
-
The ideal amount of glenoid- and humeral-sided lateralization is still unknown.
Grammont described the first commercially available and clinically successful RSA design which relied on 4principles:1–5
Medialization of joint centre of rotation (155° neck-shaft angle) to increase the deltoid lever arm
Joint centre of rotation at the bone-implant interface on the glenoid to reduce shear forces
Distalization of the humerus to retension deltoid fibers
A semi-constrained configuration to provide stability
Despite satisfactory results, Grammont’s design has several drawbacks such as instability, poor rotation, notching, loosening and loss of shoulder contour. Subsequent RSA designs have been aiming to address these limitations by lateralization, which can be humeral-sided, glenoid-sided or combined (global).
The Grammont principles were aiming for stable primary bone-glenoid implant fixation and providing good active forward elevation (AFE) and abduction. Grammont’s RSA design was associated with satisfactory but limited function with reduced rotational performance, a higher complication rate due to instability and notching, all contributing to clinician caution over the use of RSA in younger patients.3
Problems of Grammont design principles
-
Excessive medialization leading to instability due to decreased tension on the intact posterior cuff.6 This explains the higher dislocation rates of RSA with older Delta III designs,7 a problem much less observed during the last decade with increased offset designs and strategies.
-
Decreased and weak external rotation due to loss of tension of the posterior cuff6
-
Altered shoulder contour due to reduced offset and increased arm length8
-
A 155° neck-shaft angle (NSA) combined with glenoid medialization leading to high rates of scapular notching5,6 associated with polyethylene debris, macrophage, osteoclast activation, and possible loosening9–11
Design improvements with lateralization
In general implant lateralization is aiming to provide:
-
Improved stability by placing the remaining rotator cuff at its appropriate resting length and improvement in the deltoid wrapping angle (deltoid wrapping around proximal humerus)12,13
-
Improved rotation by optimizing the posterior rotator cuff length-tension relationship12
-
Reduced friction type impingement mainly in external rotation and extension, with reduced notching14,15
Lateralization of current RSAimplants and implications
Whether RSA lateralization takes place on the glenoid or humeral side has different implications. Werthel and colleagues have recently analyzed and measured 28 RSA implant combinations and 22 different implants used in current practice.16 They used the Delta III Grammont design as the reference, measuring 13.1mm of global RSA lateralization. Implants currently on the market showed variation in global lateralization (global = glenoid + humeral) ranging from 13.1mm to 35.8mm. Additionally, the authors broke up implant lateralization into humeral- and glenoid-sided. Figure 1 gives an overview of lateralization of different implants according to Werthel.16
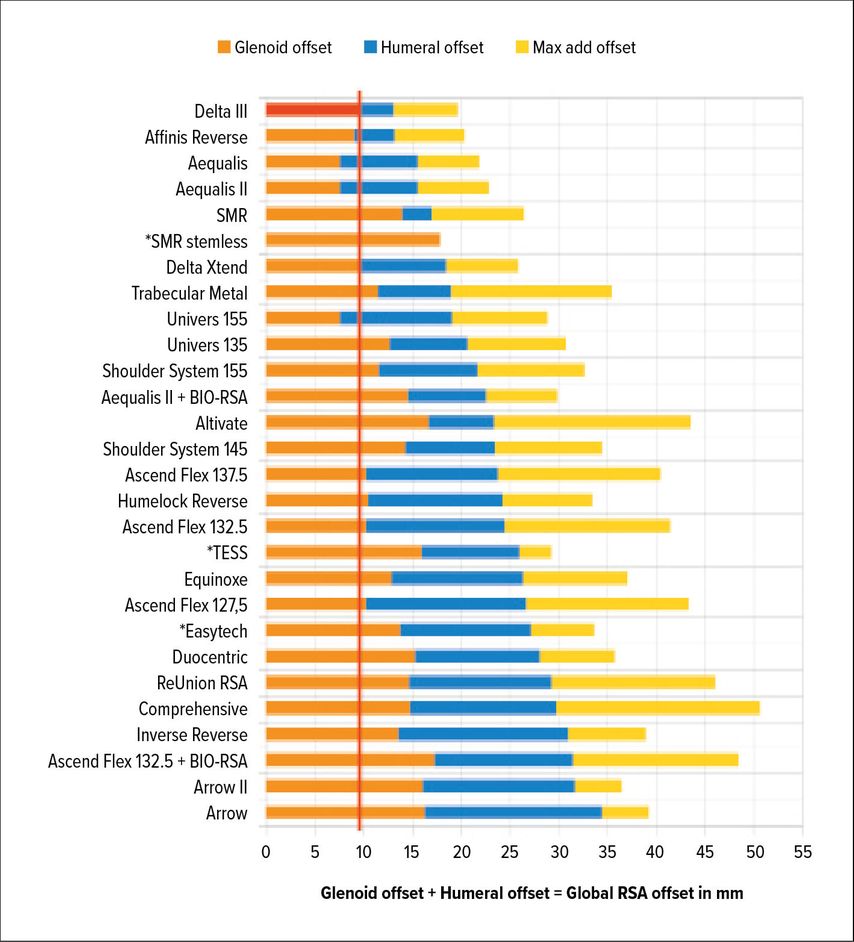
Fig. 1: Glenoid-sided lateralization from Delta III-baseline (red); yellow: max add offset due to glenosphere size/other variables according to the data by Werthel et al. 201916 (*stemless implants)
Medialized and stemless RSA
Medialized implants have less frequently been used in recent years but are still on the market. Stemless RSA (Fig. 1, marked with *) bear the risk of medialization for several reasons:
-
Humeral-sided lateralization is limited with stemless designs. Decreasing the NSA from 155° to 135° is recommended to reduce notching. An intended varus-NSA in combination with a stemless RSA may be a factor increasing the risk of periprosthetic fractures (PPF) of stemless RSA seen in unpublished cases as in Figure 2 (referred case revised at our hospital). PPF in stemless RSA were also presented by Vogt (DVSE annual meeting 2016).
-
The humeral head/neck osteotomy is intentionally high. This leads to difficult glenoid exposure. Dr Cofield frequently pointed out that the proximal humerus is shaped like a funnel. Therefore, high osteotomies or valgus osteotomies directly affect glenoid exposure with difficulties to retract the funnel shaped humeral metaphysis. Stemless RSA, therefore, bears a higher risk of high baseplate implantation or inability to implant a glenosphere of adequate size, overhang and lateralization due to challenges in glenoid exposure. Insufficient inferior overhang is not infrequently seen on radiographs of publications on stemless RSA.17 This leads to an increased risk of notching. The only available study with mid- to long-term follow-up of stemless RSA reports notching in 72% of cases at 8 years.18
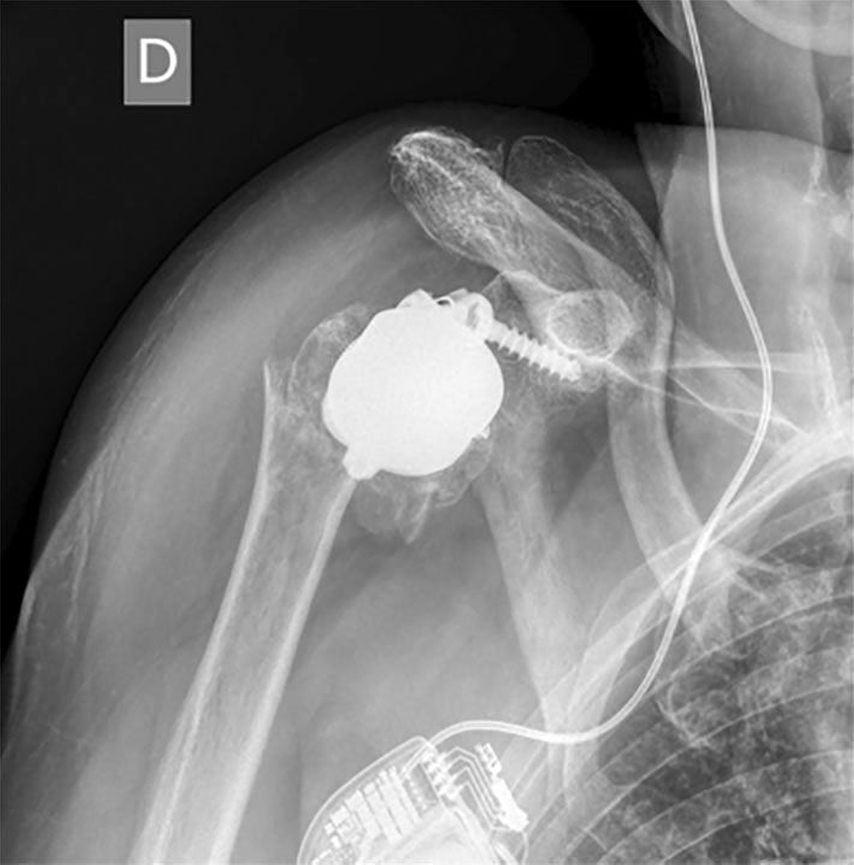
Fig. 2: Example of a periprosthetic fracture in stemless RSA
Glenoid-sided lateralization
There are implants that mainly lateralize on the glenoid. Frankle and his group were the first to lateralize with an increased offset mainly built into the glenosphere.19,20 This shifts the joint centre of rotation lateral to the glenoid bone implant interface with the risk of loosening of fixation. Glenoid-sided lateralization can occur in the base plate (Fig. 3 and 7B), in the glenosphere but also by lateralizing the base plate by a bone graft increasing the length of the scapular neck (Fig. 7A). This so-called bony-increased offset (BIO-RSA) was first described by Boileau and has a potential advantage of maintaining the distance of the joint centre of rotation to the bone implant interface after bony integration and union of the graft.21
Fig. 3: Lateralized RSA for revision of failed osteosynthesis with osteonecrosis which required burr-assisted canal opening in a younger patient. Excellent, impingement-free ROM. The radiograph shows glenoid-sided lateralization combined with humeral lateralization (145° stem which could only be implanted in slight varus due to insufficient bone stock and sclerosis). This combination illustrates the increased distance: PE insert to scapula pillar (yellow line)
Advantages of glenoid-sided lateralization
-
Reduced notching by increased impingement-free range of motion prior to contact with the lateral scapular pillar (Fig.3).15,21–23 This is a factor of major importance since notching has been shown to be associated with poorer patient satisfaction, functional outcomes, increased rate of complications and revision.24
-
Increased joint reaction forces, improved deltoid wrapping (the deltoid force vector crosses the proximal humerus due to lateralization) and stability.
Possible negative effects of glenoid-sided lateralization
-
Decreased deltoid moment arm for abduction and elevation.13 Therefore the deltoid force required for abduction increases.25
-
Possible increase of acromial stress with increased risk of acromial fractures26
-
Higher shear forces on the bone-glenoid interface with risk of loosening27
Mechanical limitations of metal-based and bony glenoid lateralization
The maximum implant-related glenoid lateralization on the market was 8.3mm, which can be increased by bone grafting as a BIO-RSA procedure.21,28 There are limitations of the offset increase and amount of bone grafting in order not to compromise secure fixation. This usually requires a bone stock of 10–15mm.
According to the authors experience, symmetric grafts of 10mm and oblique grafts of 7–13mm for glenoid bone erosion (B, C and E glenoids) can be combined with 25mm base plates with central posts (peg) and 36mm eccentric glenospheres using two additional angular stable screws as long as an inferior tilt of the base plate perpendicular to the supraspinatus fossa line is respected for balance of compressive and shear forces.
Humeral-sided lateralization
Humeral-sided lateralization can be altered by several humeral-sided modifications: First of all, the stem NSA can be reduced from 155° (Delta III) to 145° or 135°.29 It has been shown that these NSA changes from 155° to 135° only lead to an offset difference of a maximum of +3.2mm16 but notching decreases effectively with a NSA of 135° without increasing instability due to clearance of the scapula pillar as illustrated in the radiograph in Figure 3.30 Secondly, platform designs increase the humeral distance from the glenosphere leading to lateralization.13,31 The humeral implant’s onlay tray lateralization can be altered by the height of the humeral head/neck osteotomy. Additionally, the onlay types can be embedded into the metaphysis (inlay implantation reduces humeral lateralization). Finally, tray orientation and its offset as well as the angle of the polyethylene insert can lateralize the stem and humerus.
Advantages of humeral lateralization
-
Restoration of humeral position and its tuberosities tensioning the remaining cuff,32 increasing compressive joint reaction forces and stability33
-
It also increases the lateral deltoid tension, deltoid abduction leaver arm and the wrapping angle of the deltoid leading to increased stability and compressive forces.12
Disadvantages of isolated humeral lateralization
Scapular impingement and notching may not be as effectively addressed compared to increased glenoid offset or by changing the NSA from 155° to 135°, which are effectively preventing notching. The reduction of notching by changing the NSA is mainly brought about by tilting the caudal PE insert away from the scapula pillar, not by the associated minor lateralization.
Risks of lateralization
Lateralization may have advantages but there is a risk of excessive global lateralization:
-
Overstuffing in smaller patients16,34
-
Overstuffing in the presence of soft tissue contractures
Possible consequences of overstuffing:22,28,30,32
-
Difficulties reducing the joint
-
Difficulties to repair subscapularis
-
Nerve stretching
-
Reduced motion
-
Polyethylene wear
-
Acromial impingement and stress fractures
How much to lateralize?
Werthel and coauthors describe the goal of restoration as the anatomical insertion and tensioning of the remaining rotator cuff as well as restoration of an anatomical wrapping angle of the deltoid.16 To achieve this goal the authors recommend greater tuberosity lateralization at +0mm. Werthel has also shown that glenosphere-size alteration from 36mm to 42mm only changes the global offset by +1mm. It is very important to realize that increased glenoid offset leads to increased impingement-free rotation as confirmed by an experimental study by Berhouet, who used a 7mm increased glenoid offset.35 Maintaining this glenoid-sided offset changing from a 36mm glenosphere to 42mm even potentiated impingement free rotation. However, the ideal amount of global, glenoid-sided and humeral-sided lateralization and its patient-specific modification remain unknown.16
Notching: Important factor for poorer outcomes
The incidence of scapular notching ranged between 50% and 96% in numerous studies before 2010.35 It is classified according to Sirveaux (Fig.4).3 Implant position and RSA design have shown to improve rates of notching to 10–30% in recent years.11 For stemless RSA notching rates of 19% at 2 years36 and 72% at 8 years18 are concerning and need to be further investigated. Stemless humeral implants do not influence notching directly but may technically compromise adaequate base plate implantation and lateralization. In 2019 Simovitch published mid-term results of 324 patients with a 14.5% rate of notching at a minimum of 5 years.24 The authors found statistically significant worse clinical outcomes, range of motion, complications and rate of revision in patients with notching, despite low implantation of the base plate with inferior glenosphere overhang. Glenosphere size and gender did not influence notching. The implant design of the RSA in this study was globally lateralizing with 25% glenoid and 75% humeral contribution according to the classification by Werthel.16
Isolated increased glenoid offset with bone combined with older Grammont type III stems has most recently been shown to have good mid- to long-term results with 96% graft incorporation and 18% of severe notching.23 Increased glenoid offset with low base plate position, inclination, glenosphere overhang as well as decreased NSA with respect to classical Grammont design may decrease long-term rates of notching in the future.
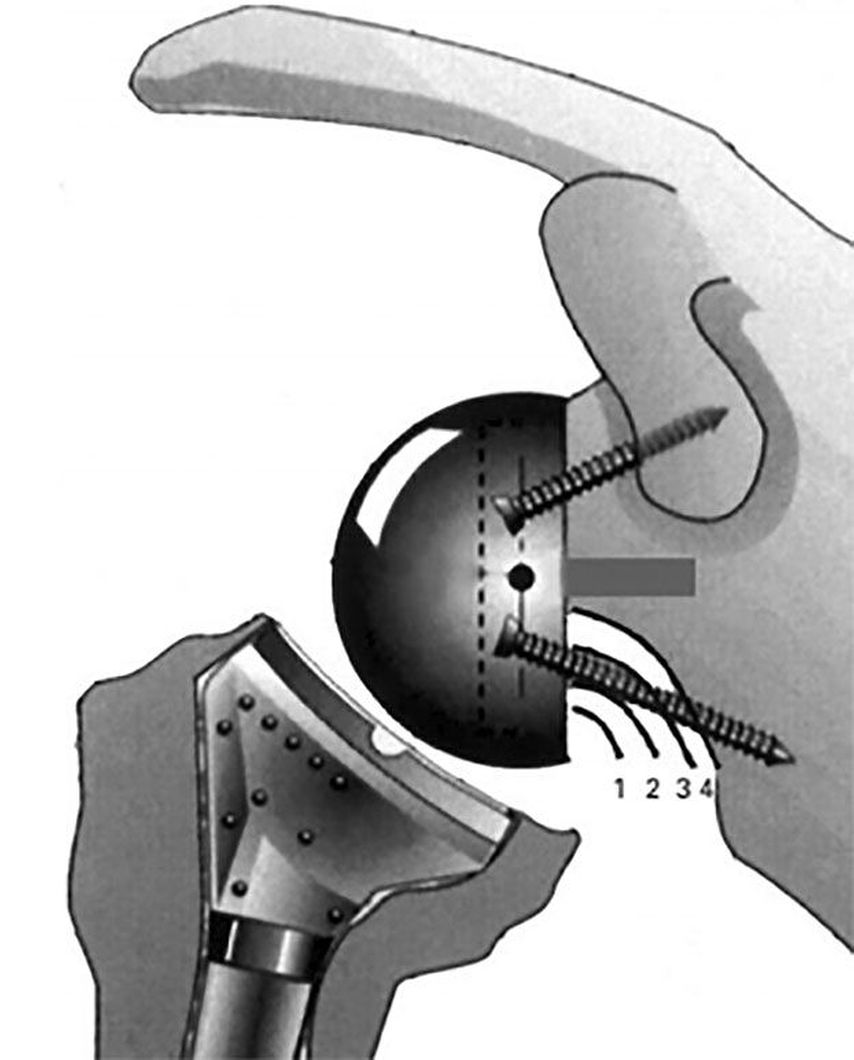
Fig. 4: Notching: classification and image according to Sirveaux et al. 20043
Patient-specific risk factors for notching
-
Shorter stature24
-
Lower body weight and BMI23,24
-
Better preoperative ROM22
-
Young age37
The authors preference derived from own experience, literature and discussion with Dr Walch and Dr Athwal
All shoulder arthroplasties are planned with a 3D software (Fig.5, BlueprintTM) allowing the dynamic simulation and evaluation of impingement-free rigid body range of motion28 for an Aequalis ascend flex stem with a Perform Reversed glenoid-sided implant (Wright).
The software displays many different parameters for preoperative planning. We want to draw attention to the following parameters which are useful for planning patient-specific lateralization:
-
Glenoid best fit sphere radius (GBFSR) is an indicator of patient, glenoid, humeral head and shoulder size (common range: 25–45mm). The authors are cautious about overstuffing for GBFSR <30mm.
-
Arm change – lateralization: caution with lateralization beyond +10mm
-
Arm change – distalization (variation: 20–40mm): Caution with distalization beyond -40mm
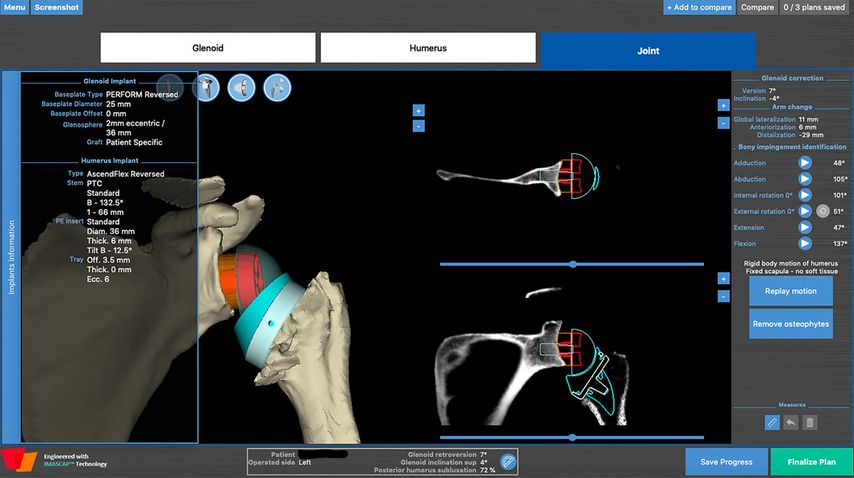
Fig. 5: Example of dynamic preoperative planning of rigid body ROM aiming to prevent notching (BlueprintTM)
The authors (SB) case series (n>100, not yet published) shows that virtual friction-type impingement-free external rotation and extension to >40° can in most cases only be achieved by increased glenoid offset, which can be metal (+2mm inbuilt in Perform Reversed base plate with +3mm or +6mm augmentation) or bony (±2mm inbuilt in base plate with standard BIO-RSA of +7mm or +10mm or oblique BIO-RSA of 7–13mm or patient-specific BIO-RSA of variable height) in combination with an eccentric 36mm +2mm glenosphere.
Glenoid side
The authors preference in most cases is a bony-increased or metal-increased offset RSA (BIO- or MIO-RSA of +3–10mm) preferably with a 25mm base plate (Perform Reversed with +2mm) implanted low on the glenoid and perpendicular to the supraspinatus fossa line according to Boileau’s recommendations,28 combined with a 36mm eccentric (+2mm) glenosphere. This strategy is aiming to reduce friction-type notching for extension and external but also internal rotation.14,35
Gap space assessment
If the humeral osteotomy is considered to be already low and definitive, the amount of possible glenoid-sided lateralization can be judged by a 20/40mm spacer block. The required space for reduction (40mm) between the reamed glenoid and the humeral osteotomy can be broken up into +2mm (standard base plate inbuilt lateralization) +18mm (36 GS) +20mm humeral insert/tray jump distance as illustrated in Figure 6. With the insertion of a 40mm spacer paddle, the gap tension can be evaluated estimating possible additional lateralization with metal-increased offset (optional augmentation of +3mm or +6mm) or bony increased offset with a standard Perform Reversed base plate (+7mm or +10mm bone). These absolute gap space values are subject to change for different implant sizes and different manufacturers but can be calculated.
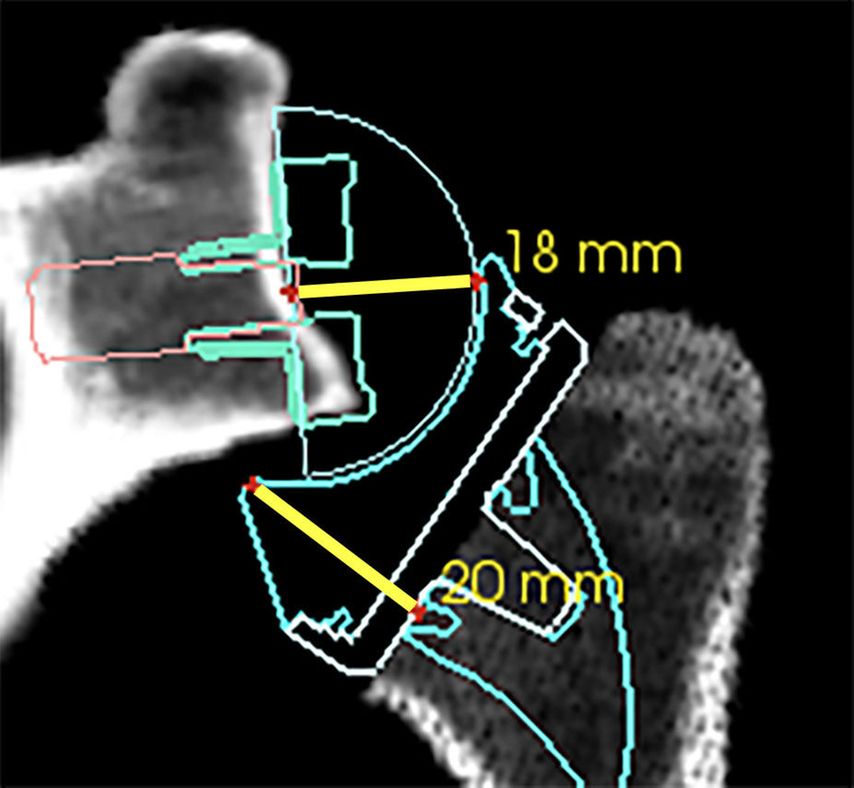
Fig. 6: Gap space requirement for reduction of a standard Perform Reversed 36mm GS with a standard insert (+6mm) and tray (+0mm). Base plate/GS distance of approximately +2mm +18mm and insert/tray jump distance of 20mm
Humeral side
The humerus is adapted to increased glenoid offset. This often requires a lower osteotomy on the humerus resulting in seating of the tray in the metaphysis as an inlay technique. We aim for an inlay depth so that the highest point of the tray is at level with the greater tuberosity, according to Dr Walch. Due to a low osteotomy we use a +3.5mm tray which is dialed to position 6. This medializes the humerus by 3mm (additional tray offset of +2mm medializes the humerus; +1mm for dialing to position 6). Once the glenosphere is implanted, the required amount of humeral resection can be judged further with a 20mm spacer block, according to Dr Athwal and as seen in Figure 6. It should be insertable between the glenosphere and the humeral osteotomy under muscle relaxation. Ability of insertion of the crossed index and middle finger or the back side (18mm) of the standard white plastic Prosthesis Reducer (Aequalis Perform Reversed standard glenoid instrument MWJ100) are also good estimates. Recently a company has launched humeral trial inserts with integrated sensors for contact pressure measurements (joint reaction force), which can be used during surgery. To date the ideal amount of pressure as a joint reaction force and tension of the deltoid after reduction in a patient under general anesthesia and muscle relaxation are unknown.
Risks
Despite the strategy of humeral compensatory resection, reduction of the arthroplasty can still be difficult with increased glenoid offset (+2mm with +3 or +6mm MIO-RSA, +10mm BIO-RSA) especially if no initial gap assessment between glenoid and humerus was undertaken. Tensioning with the plastic Prosthesis Reducer (MWJ100) to overcome the “jump distance” of 20mm of the insert concavity (Fig. 6) for reduction onto the glenosphere and removal of the arm out of the armholder for reduction are at times helpful and necessary. In rare cases it can occur that joint reduction is impossible and the required compensatory humeral osteotomy would be too low at the humeral calcar. This rare situation can only be solved by intra-operative removal of the glenosphere and base plate with reduction of the glenoid or base plate offset. The osteotomy should also never compromise the posterior rotator cuff insertion. To avoid postero-lateral over-resection with posterior rotator cuff compromise by a low humeral osteotomy, inlay reamers have been designed to protect the anterior but most importantly the postero-superior humeral cortex and its postero-superior rotator cuff insertion. Overstuffing should be avoided for the aforementioned reasons.
Authors patient-specific RSAlateralization strategies for two patient groups
Group I: Elderly patients over 70 years of age, less active, with comorbidities, increased BMI and shorter life expectancy
They may not require maximal increased glenoid offset RSA with ideal impingement-free rotation and extension at the cost of increased tension, risk of joint overstuffing with increasing the risk of nerve distension, pain and acromion or scapular spine fractures in more osteoporotic patients. In these patients the author prefers low implantation of the base plate with minimal increased offset and an eccentric glenosphere, even if the 3D analysis shows extension and external rotation values below 35°.
Group II: Younger patients below 70 years of age who are active and slim
They may well benefit from increased glenoid offset RSA with a larger range of impingement-free extension and rotation preventing friction type notching and mid- to long-term glenoid loosening by osteolysis. This strategy may provide the best prognosis for increased RSA longevity in younger and more active patients with longer life expectancy and slim patients which have been shown to require increased ROM. The humeral resection and osteotomy is therefore adapted to the ideal glenoid offset aiming for a larger impingement-free ROM with extension and external rotation over 40°. Figure 7 shows examples of BIO- and MIO-RSA.
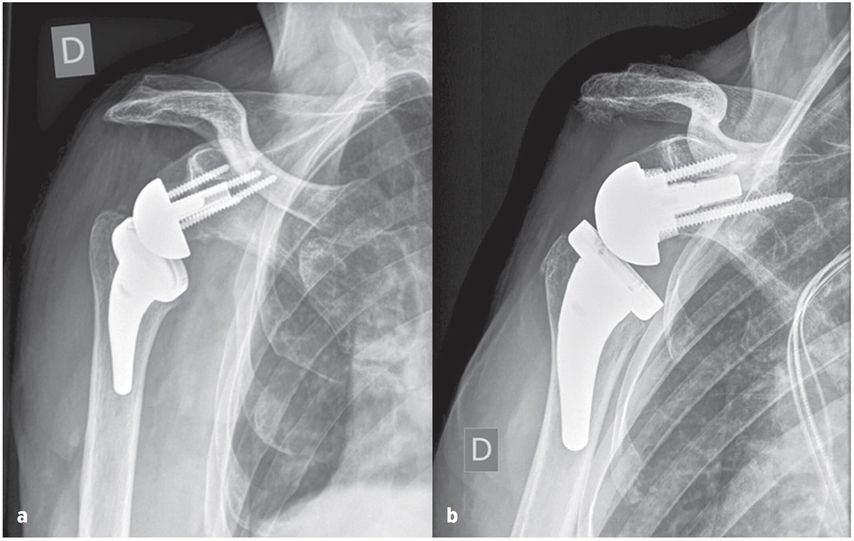
Fig. 7: True ap radiographs of glenospheres: a) bony-increased offset, b) metal-increased offset
References:
1 Baulot E et al.: [Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases]. Acta Orthop Belg 1995; 61(Suppl 1): 112-9 2 Grammont PM et al.: Etude et réalisation d’une nouvelle prothèse d’épaule [Internet]. 1987 [cited 2020 Jul 28]. Available from: /paper/Etude-et-r%C3%A9alisation-d%27une-nouvelle-proth%C3%A8se-Grammont-Trouilloud/ce86490e649299d28303798d257d14c9b2642fbe 3 Sirveaux F et al.: Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004; 86(3): 388-95 4 Naveed MA et al.: The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br 2011; 93(1): 57-61 5 Werner CML et al.: Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005; 87(7): 1476-86 6 Boileau P et al.: Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005; 14(1 Suppl S): 147S-161S 7 Wall B et al.: Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007; 89(7): 1476-85 8 Henninger HB et al.: Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 2012; 21(9): 1128-35 9 Nyffeler RW et al.: Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br 2004; 86(8): 1187-91 10 Simovitch RW et al.: Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am 2007; 89(3): 588-600 11 Friedman RJ et al.: Scapular notching in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2019; 27(6): 200-9 12 Routman HD et al.: Reverse shoulder arthroplasty prosthesis design classification system. Bull Hosp Jt Dis (2013) 2015; 73(Suppl 1): S5-14 13 Hamilton MA et al.: Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res 2015; 33(4): 605-13 14 Lädermann A et al.: Scapular notching on kinematic simulated range of motion after reverse shoulder arthroplasty is not the result of impingement in adduction. Medicine (Baltimore) 2015; 94(38): e1615 15 Valenti P et al.: Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res 2011; 469(9): 2550-7 16 Werthel JD et al.: Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop 2019; 43(10): 2349-60 17 Ballas R, Béguin L: Results of a stemless reverse shoulder prosthesis at more than 58 months mean without loosening. J Shoulder Elbow Surg 2013; 22(9): e1-6 18 Beck S et al.: Long-term results of the reverse Total Evolutive Shoulder System (TESS). Arch Orthop Trauma Surg 2019; 139(8): 1039-44 19 Frankle M et al.: The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. a minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am 2006; 88(Suppl 1 Pt 2): 178-90 20 Mulieri P et al.: Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am 2010; 92(15): 2544-56 21 Boileau P et al.: Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011; 469(9): 2558-67 22 Katz D et al.: Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int Orthop 2016; 40(1): 99-108 23 Boileau P et al.: Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years’ follow-up. J Shoulder Elbow Surg 2020; 29(10): 2111-22 24 Simovitch R et al.: Impact of scapular notching on reverse total shoulder arthroplasty midterm outcomes: 5-year minimum follow-up. J Shoulder Elbow Surg 2019; 28(12): 2301-7 25 Giles JW et al.: Implant design variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res 2015; 473(11): 3615-26 26 Wong MT et al.: Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg 2016; 25(11): 1889-95 27 Harman M et al.: Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 2005; 14(1 Suppl S): 162S-167S 28 Boileau P et al.: Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management of glenoid bone loss and erosion. J Shoulder Elbow Surg 2017; 26(12): 2133-42 29 Lädermann A et al.: Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop 2015; 39(11): 2205-13 30 Erickson BJ et al.: The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2015; 24(6): 988-93 31 Kirsch JM et al.: Platform shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2018; 27(4): 756-63 32 Franceschetti E et al.: The role of the subscapularis tendon in a lateralized reverse total shoulder arthroplasty: repair versus nonrepair. Int Orthop 2019; 43(11): 2579-86 33 Langohr GDG et al.: The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg 2015; 24(6): 972-9 34 Boileau P, Walch G: The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br 1997; 79(5): 857-65 35 Berhouet J et al.: Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23(2): 151-8 36 Teissier P et al.: The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: clinical and radiographic results. J Shoulder Elbow Surg 2015; 24(1): 45-51 37 Ernstbrunner L et al.: Reverse total shoulder arthroplasty for massive, irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am 2017; 99(20): 1721-9
Das könnte Sie auch interessieren:
Mehr kardiovaskuläre Ereignisse und Malignome?
Mit Tofacitinib, einem Strukturanalogon von ATP, wurde 2013 erstmals ein Januskinase-Inhibitor (JAKi) in der Schweiz zugelassen. Die Vertreter dieser Medikamentenklasse haben sich gut ...
Seltene Kleingefässvaskulitiden im Fokus
Bei Vaskulitiden der kleinen Gefässe liegt eine nekrotisierende Entzündung der Gefässwand von kleinen intraparenchymatösen Arterien, Arteriolen, Kapillaren und Venolen vor. Was gilt es ...
Elektive Hüft-TEP bei Adipositas Grad III
Übergewichtige Patient:innen leiden früher als normalgewichtige Personen an einer Hüft- oder Kniearthrose. Allerdings sieht die aktuelle S3-Leitlinie zur Behandlung der Coxarthrose in ...