Emerging oncological indications to liver transplantation
Author:
Dre méd. Giulia Magini
Service de gastro-entérologie et d’hépatologie, HUG, Hôpitaux Universitaires de Genève
E-mail: giulia.magini@hcuge.ch
Vielen Dank für Ihr Interesse!
Einige Inhalte sind aufgrund rechtlicher Bestimmungen nur für registrierte Nutzer bzw. medizinisches Fachpersonal zugänglich.
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
The use of liver transplantation (LT) for oncological indications has recently expanded from hepatocellular carcinoma (HCC) to other primary hepatobiliary malignancies such as cholangiocarcinoma (CCA) and secondary malignancies including neuroendocrine and colorectal metastases. This expansion has been made possible through a careful patient selection that has allowed to achieve superior oncological outcomes with respect to available treatments and compelling overall survival compared to non-oncological indications to LT. Indeed, given organ shortage, as for other indications, the benefit for the single patient must be balanced with an ‘acceptable’ expected survival, set to at least 70% at 5 years. The aim of this article is to review the present selection criteria for LT in the case of colorectal liver metastases (CRLMs), neuroendocrine tumors (NETs) and cholangiocarcinoma to guide practitioners in timely referringeligible patients to transplantation centers.
Keypoints
-
The standard of care for cholangiocarcinoma and liver metastasis from gastrointestinal tumors is liver resection preceded by neoadjuvant therapy, but this is only possible in a small proportion of patients.
-
Liver transplantation is an alternative for non-resectable tumors, in highly selected patients with clinic, radiological and pathological features in favor of a non-aggressive tumor biology.
-
Molecular profiling, liquid biopsy and target systemic therapies offer a prospect for the selection of the best candidate and treatment individualization.
-
Strategies to expand the donor pool must be implemented in order to minimize the impact of those novel indications on the waiting list.
Liver metastases from colorectal carcinoma
Colorectal carcinoma (CRC) is the third most common malignancy world-wide.1 At the time of diagnosis, only 20% of patients are resectable, but even with resection, survival remains poor, with high disease-recurrence rates and 5-year overall survival (OS) of only 30–40%.2 The liver is the most frequent site of metastatic CRC, and margin negative liver resection (LR) along with systemic and locoregional therapies (LRT) offer the only chance for a cure. Patients with CRLMs not amenable to resection have a dismal survival (5–10% at 5 years). Liver transplantation (LT) has been offered as savage therapy but, because of inadequate patient selection, resulted in poor outcomes in early experiences.3
In Norway, a country with excellent donor organ access and a median wait time for LT of 2 months, the SECA-I and SECA-II trials have offered a reappraisal of the role of LT for unresectable CRLM.
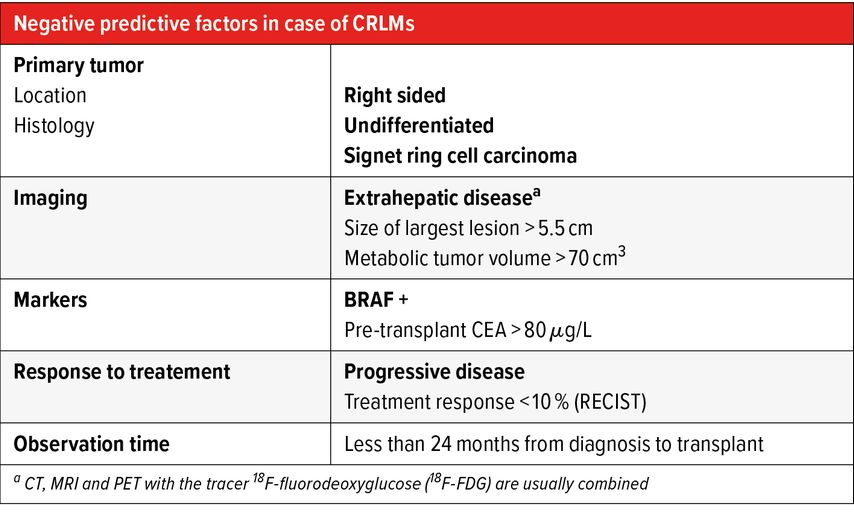
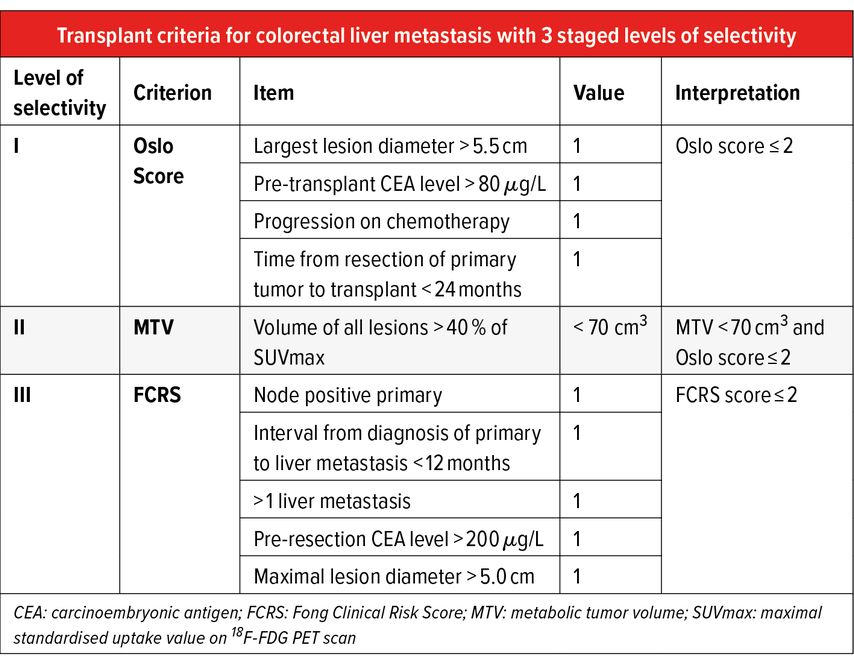
The SECA-I trial, a prospective pilot trial, included highly selected recipients who have liver-limited disease with complete oncologic resection of their primary tumor and at least six weeks of pre-transplant chemotherapy. A 5-year OS of 60% was achieved, compared to 10% with palliative chemotherapy. 100% of patients presented recurrent disease 2 years after LT. Poor prognostic factors included tumor size exceeding 5.5cm, LT within 2 years of the index colorectal cancer surgery and an elevated carcinoembryonic antigen (CEA) >80μg/L.4
The subsequent SECA-II trial was based on stricter selection criteria and included 15 patients with a minimum of 10% response to systemic therapy, at least 1 year between the diagnosis of the primary tumor and LT listing, no lesion over 10cm before chemotherapy and, if more than 30 lesions were present, all had to be <5cm and patients required at least 30% response to chemotherapy by RECIST criteria. OS was 100%, 83% and 83% for 1, 3, and 5 years respectively. The authors reported a disease-free survival (DFS) of 53%, 44% and 35% at 1, 2 and 3 years post-LT.5
Importantly in both studies about 70% of all recurrences were small lung metastases which displayed a slow growth, as in non-immunosuppressed patients, and were mostly amenable to curative resection.
Owing to the high recurrence rate, three randomized trials comparing transplantation to chemotherapy (Transmet study, NCT02597348; SECA-III study, NCT03494946; and COLT study, NCT03803436) are currently recruiting and will better delineate the oncological impact of LT over standard-of-care therapy.
To resume, early referral to a liver transplantation center must be evoked in case of
-
radically resected primary lesion, ideally in the absence of lymph node involvement;
-
absence of extrahepatic metastases;
-
absence of negative prognostic factors (Tab. 1);
-
sustained treatment response and a time from resection of the primary tumor to transplant of at least 2 years as surrogate markers of a non-aggressive tumor biology.
Tab. 1: Negative predictive factors for liver transplantation (LT) in case of colorectal liver metastasis (CRLMs). In bold relative contraindications to LT
A stage approach to patient selection can be used based on two available scores, made up by the combination of the above risk factors, plus the metabolic tumour volume (MTV) on pre-transplant PET-CT, calculated as the total enhancement volume in the lesions with an uptake exceeding 40% of standardized uptake volume (Tab. 2).4,6
Neuroendocrine tumors liver metastases
Gastroenteropancreatic NETs (GEP-NET) represent 60% of all NETs and at the time of diagnosis, there are synchronous metastases in already 21–55% of the patients, of which 72–82% are in the liver.7
Once the primary tumor has been resected, the treatment of choice for neuroendocrine liver metastases (NELM) is LR who affords significant 5-year survival improvement compared with chemotherapy and embolization (61–74%).8,9 However, less than half of the patients are eligible for resection, and recurrence rates after resection are high (68–76% at 3 years, 84–94% at 5 years).8,9
Most of the studies evaluating outcomes of LT for neuroendocrine metastasis are retrospective in nature. A systematic review conducted by Moris et al. in 2017 summarized 64 studies evaluating the outcomes of liver transplant for neuroendocrine tumor liver metastases. The overall recurrence rate after liver transplant ranged from 31.3% to 56.8%. The 5-year overall survival rate ranged from 50% to 70.7%.10
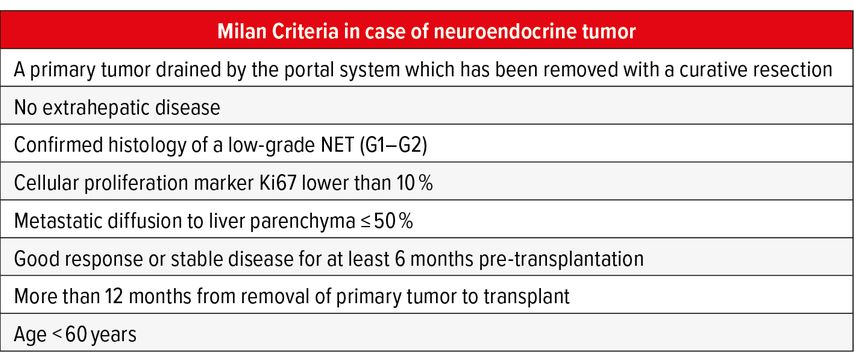
Mazzaferro et al., based on the results of a retrospective study on a cohort of 24 patients who achieved a 5-year OS and DFS of 90% and 77% respectively established eligibility criteria for LT resumed in Table3. When strictly applied in prospective studies, those criteria resulted in significant advantages compared to the non-transplant group at 5 and 10 years (OS LT 97.2% and 88.8% vs non-LT 50.9% and 22.4% respectively) with only 13% recurrence rate.11,12
Those criteria, the so called ‘Milan Criteria in case of Neuroendocrine Tumor’ are adopted by many centers to guide candidate selection. Given the low impact of disease recurrence on survival due to the indolent growth of these tumors, the indication for LT must be evaluated on a case-by-case basis taking in to account the oncological benefit of the single patient and the local waiting list.
Two other negative prognostic factors that must be taken into account are primary tumor in the pancreas and symptomatic tumor.
A multimodal approach including LRT like transarterial chemoembolization or radioembolization and systemic therapies like somatostatin analogues, peptide receptor radionuclide therapy, everolimus and sunitinib can play a pivotal role in disease control in the neoadjuvant and adjuvant setting. New biomarkers, including peptides/growth factors, DNA/RNA markers and circulating tumor cells are currently under investigation to detect minimal extra-hepatic NET disease, responsible for late recurrences and evaluate treatment effect. The role of emerging imaging techniques like Ga-68 dotatate PET-CT for earlier and more precise tumor detection must be validated and implemented in prospective trials.
Cholangiocarcinoma
Cholangiocarcinoma is a malignant tumor of the biliary system and is the second commonest primary liver cancer after hepatocellular carcinoma (HCC). Depending on its location, it can be classified as perihilar cholangiocarcinoma (pCCA), intrahepatic cholangiocarcinoma (iCCA) or distal cholangiocarcinoma (mid-third and lower third of the bile duct).
Perihilar cholangiocarcinoma
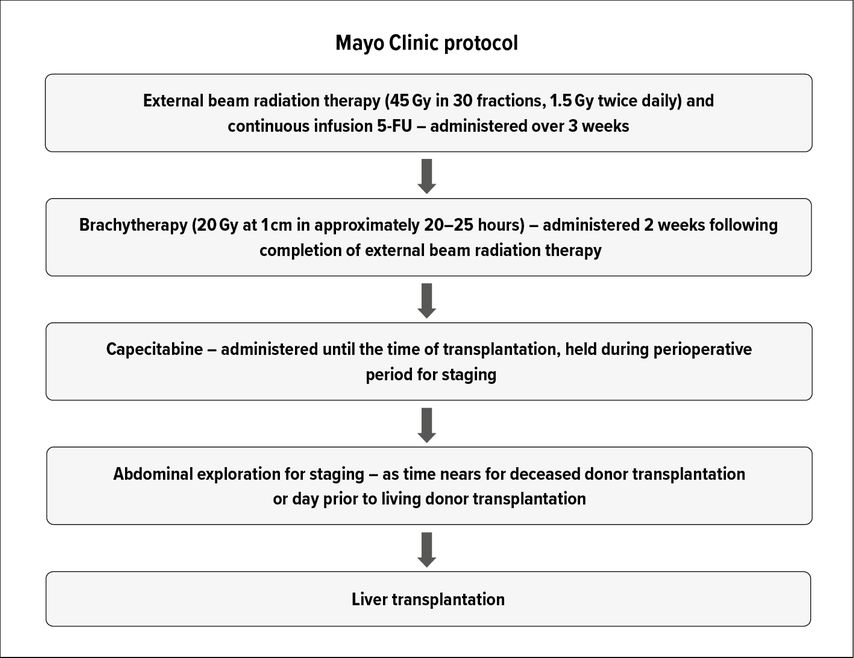
LR represents the gold standard for treatment. However, only a minority of patients (ca. 25%) are eligible for LR.13 In addition LR results in low margin negativity (25–40%), high recurrence (up to 76%) and low survival rates (5-year survival 30–40%).13,14 While early results for LT in pCCA demonstrated dismal outcomes, the development of neoadjuvant chemoradiotherapy protocols along with stringent patient selection has yielded excellent oncological outcomes.15,16 The neoadjuvant chemoradiotherapy regimen typically involves external beam radiotherapy and brachytherapy with 5-fluorouracil as radiosensitizer, followed by oral capecitabine until LT (Fig. 1). Patients are excluded if the tumor diameter exceeds 3cm in radial axis, the CCA is located below the cystic duct, any tumor plane violation (such as after biopsy or surgical exploration) or have presence of lymph node or distant disease.
In a large multi-institutional consortium, including 216 patients, 5-year recurrence-free and intention-to-treat survival rates were 65% and 53% respectively. The favorable oncological results have led pCCA to become an accepted indication for LT, as long as selection criteria and neoadjuvant treatment protocols are strictly adhered to.17
To clarify the role of LT in resectable disease, the ongoing multicenter prospective randomized trial known as TRANSPHILL (NCT02232932) compares neoadjuvant chemoradiotherapy and LT with standard-of-care liver and bile duct resection for patients with resectable pCCA.
The role of neoadjuvant therapy for downstaging pCCA that exceeds currently accepted LT criteria remains to be elucidated.
Intrahepatic cholangiocarcinoma
LR remains the mainstay of treatment for intrahepatic cholangiocarcinoma (iCCA) but many patients present with advanced disease, which limits therapeutic options.
Unfortunately, even with margin-negative resection, 5-year survival is low, ranging between 30% and 40%, often adversely affected by high disease recurrence rates and low cure rates.7
Because of the very poor outcomes in the early experience of liver transplant for iCCA, this disease is accepted as indication only in the context of prospective controlled trials.
Indeed, retrospective studies analyzing outcomes of LT for iCCA incidentally discovered or misdiagnosed as HCC, revealed that patients with solitary nodules ≤2cm in diameter without vascular invasion, achieve 5-year OS of 62%. When compared to HCC outcomes, disease recurrence rates remained significantly higher for cholangiocarcinoma. Vascular invasion and incomplete response to pre-transplant LRT were independently associated with recurrence risk.18
The favorable outcomes noted in the abovementioned retrospective reviews have provided the basis for the ongoing prospective trial from University Health Network and Hospital Clinic of Barcelona. This trial will evaluate outcomes of LT for cirrhotic patients who have biopsy-proven very early iCCA (≤2cm) with a low CA19-9 level.
Small series reporting results of LT for more advanced tumors showed that sustained response to neoadjuvant therapy, a surrogate for tumor biology, has the strongest predictive power irrespective to original tumor size but the role of LT should be assessed in prospective trials.19,20
As neoadjuvant therapy use grows, standardization of both systemic therapies and LRT is necessary to generate the most meaningful evidence for the development of treatment protocols. Moreover, the incorporation of circulating tumor DNA analysis in the treatment decision process may identify poor candidates that would not benefit from surgical interventions. Through genomic analyses, molecular subclasses of CCA can be identified, and may serve as potential targets for future therapeutic strategies.
Conclusions and future perspectives
Revising allocation policies to incorporate oncological indications other than HCC will be challenging because of organ shortage.
Two endpoints should be targeted to justify the use of the limited resource of donated organs for cancer patients with expanded indications. First, any expansion must be supported by competitive overall survival with respect to non-transplant indications. Second, LT must lead to a significant gain in survival compared to non-LT options. For both those purposes, LT transplantation for CAA or secondary liver tumors should be implemented in the context of prospective trials with standardized aggressive neoadjuvant protocols integrating a multimodal approach and stringent selection criteria.
At present markers of tumor biology, response to pre-transplant therapy and disease stability over time provide the best risk stratification for transplantation.
Emerging tools like genomics and immunogenomics (the interaction between the tumor and the immune system), liquid biopsy or circulating fragments of tumor genome may optimize patient risk stratification by defying aggressiveness and real tumor load.
Expansion of the donor pool with extended criteria donors, partial liver transplantation and living donors could be considered to ensure transplantation in an often narrow therapeutic window.
Literature:
1 American Cancer Society: Key statistics for colorectal cancer (accessed April 23, 2021). Available from: https://www.cancer.org/cancer/colon-rectal-cancer/about/keystatistics 2 Kanas GP et al.: Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012; 4: 283-301 3 Penn I: Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991; 110: 726-35 4 Hagness M et al.: Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg 2013; 257: 800-6 5 Dueland S et al.: Survival following liver trans-plantation for patients with nonresectable liver-only colorectal metastases. Ann Surg 2020; 271: 212-8 6 Fong Y et al.: Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309-18 7 Moeckli B et al.: Recent developments and ongoing trials in transplant oncology. Liver Int 2020; 40: 2326-44 8 Mayo SC et al.: Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010; 17: 3129-36 9 Sarmiento JM et al.: Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003; 197: 29-37 10 Moris D et al.: Liver transplantation in patients with liver metastases from neuroendocrine tumors: a systematic review. Surgery 2017; 162: 525-36 11 Mazzaferro V et al.: Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol 2007; 47: 460-6 12 Mazzaferro V et al.: The long-term benefit of liver transplantation for hepatic metastases from neuroendocrine tumors. Am J Transplant 2016; 16: 2892-902 13 Nagino M et al.: Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013; 258: 129-40 14 Groot Koerkamp B et al.: Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg 2015; 221: 1041-9 15 Sudan D et al.: Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002; 2: 774-9 16 Rea DJ et al.: Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005; 242: 451-61 17 Darwish Murad S et al.: Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012; 143: 88-98 18 Sapisochin G et al.: Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016; 64: 1178-88 19 Lunsford KE et al.: Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018; 3: 337-48 20Wong M et al.: Downstaging locally advanced cholangiocarcinoma pre-liver ransplantation: a prospective pilot study. J Surg Res 2019; 242: 23-30
Das könnte Sie auch interessieren:
Tachycardie supraventriculaire
Les tachycardies paroxystiques supraventriculaires régulières apparaissent généralement chez des patients sans cardiopathie structurelle. Dans cet article, nous discutons de l’importance ...
Risques du tabagisme pour la santé – sevrage tabagique
«Un fumeur avide qui lit et relit l’importance des risques du tabagisme pour sa santé cesse dans la plupart des cas ... de lire», avait déclaré Winston Churchill. «Le tabac est le seul ...
Diabète et foie
De nombreuses personnes souffrant d’obésité ou de diabète de type 2 (DT2) développent au cours de leur vie une maladie hépatique stéatosique associée à un dysfonctionnement métabolique ( ...