Allergies: Prevention, risk factors and recommendations
Authors:
Isabella Pali-Schöll, PhD, MDSci.1,2
Yvonne Lamešić, cand. med.1
1Interuniversity Messerli Research Institute
University of Veterinary Medicine
Vienna
2Institute of Pathophysiology and Allergy Research
Medical University of Vienna
E-Mail: Isabella.pali@meduniwien.ac.at
Sie sind bereits registriert?
Loggen Sie sich mit Ihrem Universimed-Benutzerkonto ein:
Sie sind noch nicht registriert?
Registrieren Sie sich jetzt kostenlos auf universimed.com und erhalten Sie Zugang zu allen Artikeln, bewerten Sie Inhalte und speichern Sie interessante Beiträge in Ihrem persönlichen Bereich
zum späteren Lesen. Ihre Registrierung ist für alle Unversimed-Portale gültig. (inkl. allgemeineplus.at & med-Diplom.at)
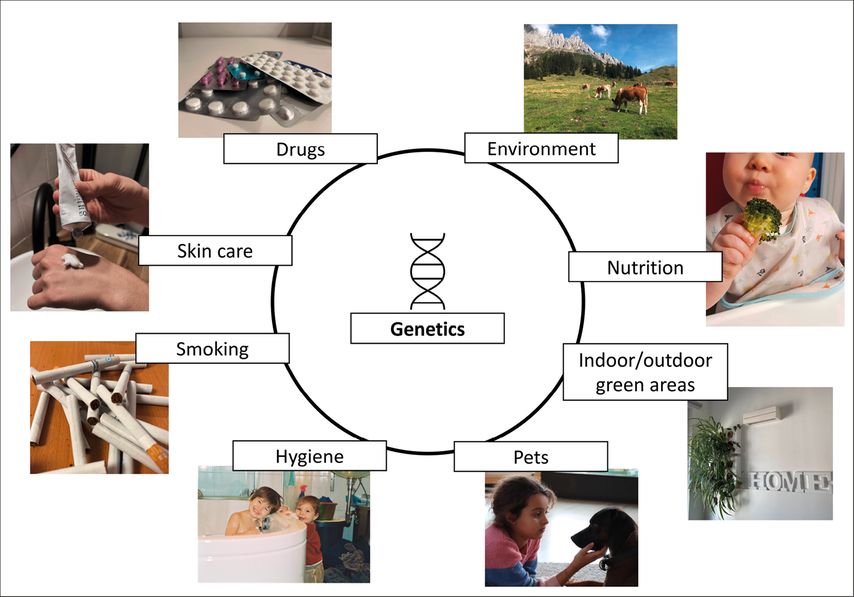
The development and severity of allergic diseases, such as allergic rhinitis, asthma and food allergies, are influenced by multiple risk factors, as displayed in Figure 1. Some of those are unalterable, like hereditary factors, while others are modifiable lifestyle choices that can directly shape the immune system – best case, towards an anti-allergic state. The present article touches upon the most important of them.
Keypoints
-
Biodiverse playgrounds and enriched indoor environment help shape a diverse microbiome.
-
Unprocessed and home-made food, including vegetables, fruits, fibers and fermented food,contribute to a lower risk for allergies.
-
Life on a farm and oral application of carrier proteins with ligands like vitamins, metals and trace elements minimize the risk of asthma and allergydevelopment and symptoms of already existing allergies.
Fig. 1: The influence factors for development and severity of asthma and allergic diseases are manifold, however, they also present a palette of potential starting points to avoid disease development
Genetics and smoking
Two well-known and frequently mentioned impact factors that both play an important role in allergic diseases are smoking and genetics. Children with allergic parents have a higher risk for developing allergies themselves, implying the existence of genetic predispositions for sensitization and allergy development.1,2 While children from non-allergic families have a 10% risk for allergy development, the risk for children with two allergic parents increases to 70%. As an exogenous risk factor, primary smoking unsurprisingly harms the overall health, and is also associated with a higher risk for asthma development, and a stronger resistance to corticosteroids as part of the asthmatic treatment.3 When it comes to second-hand smoking, maternal and also paternal smoking during pregnancy significantly increases the child’s risk for asthma.4
There are several other reasons causing allergy development and progression that can actively be manipulated to help prevent sensitization or symptoms, or at least reduce them. The following findings represent a practical aspect of allergy prevention strategies aiming to support doctors and allied health care professionals, patients and their caretakers.
Drugs
Antibiotics and gastric acid inhibitory drugs strongly interfere with proper digestion as well as the human gut microbiota,5–11 which, depending on its composition, can promote allergies and atopic diseases.12,13
Due to their impact on gut bacteria, the pH of the intestine, and the stability of orally consumed antigens, gastric acid inhibitors shift the intestinal system towards a more allergenic state, meaning that a sufficient protein degradation is prohibited due to increased pH in gastric fluid, and the immune system is set on alert due to bigger protein remnants. Further, the composition of the gut microbiome is influenced by the different chime (food remnants, pH), and may result in a disadvantageous gut environment for allergies by inducing pro-inflammatory cytokines and cells.10,11,14 This correlation was underlined by a recent analysis that showed a drastic increase in prescriptions for anti-allergic medication following gastric acid inhibitors prescriptions in Austria.6
The intake of antibiotics during pregnancy is associated with a higher risk for developing asthma in early childhood,15,16 which may also be linked to the changes in the microbiome.17 In many cases the intake of these drugs is crucial to prevent life-threatening events, but they should only be prescribed when a concrete medical indication is assured (disease ascertained by diagnosis), and intake should be restricted to the duration absolutely needed. Therefore, patients need to be clearly instructed as to when exactly the antibiotic or anti-acid drug has to be tapered and stopped. Furthermore, patients should be informed on how to maintain and especially re-establish a balanced microbiome, e.g. by a diverse plant-based and fiber-rich diet, by support with probiotic/synbiotic substances, or by diversity in their living environment.
Environmental biodiversity
A way to keep the microbiota on our skin and our mucosal surfaces (incl. the gut) balanced is to enrich the biodiversity in every-day life. This can support the immune system to recognize and attack harmful pathogens and to avoid overreacting to harmless environmental antigens in the form of allergic immune responses. Along this line, a recent 2-years study showed that introducing biodiverse playgrounds like forest floors and sod in daycare yards resulted in a greater bacterial richness, especially Gammaproteobacteria, Negativicutes and Bacilli, of children’s skin and saliva compared to those in conventional daycare centers with only limited access to organic materials.18 The skin swabs analysis from the intervention group also confirmed a decrease of potentially pathogenic bacteria, such as Haemophilus parainfluenzae, Streptococcus sp., and Veillonella sp. In addition, in the stool samples less Clostridium sensu stricto was registered after intervention in comparison to children from standard daycares.
Similar effects were found among three- to five-year-old children that were exposed to sand enriched with microbially diverse soil.19 Their skin microbiota shifted towards the composition of the enriched sand, including Gammaproteobacteria. Further, the cytokine constellations in the serum shifted towards an anti-allergic state, resulting in an increased ratio of IL-10:IL-17A (anti-inflammatory:pro-inflammatory) only in the intervention group. In parallel, the gut proteo-bacterial diversity decreased in the intervention group. Overall, these results certainly imply the relevance of direct contact with biodiverse soil in immune-regulating processes.
Apart from childhood age, such interventions might also work in adults, as an experiment with green indoor walls in office rooms revealed a growth of Lactobacillus spp., a commensal acting against pathogens and inflammation, on the skin within only four weeks of intervention.20 On top of that, the proinflammatory cytokine IL-17A levels in the serum dropped more in the intervention group with contact to green indoor walls than in the control without access to such walls. Anti-inflammatory TGF-β1 serum levels increased in both of the intervention groups, but decreased in the control groups after 28 days. Having pets represents another way to enlarge the biodiversity at home. Contact with dogs, especially during the first year of life, reduces the risk for asthma, allergic rhinitis or atopic sensitization, while cats only did that for atopic eczema.21 It was also shown that a higher number and higher diversity of pets (dogs, cats) was inversely associated with allergic manifestations (asthma, allergic rhinoconjunctivitis, or eczema) in children at the age of seven to nine years.22
Hygiene and detergents
When speaking of pets, it also needs to be taken into consideration that dogs’ coats carry mites and therefore relevant allergens that humans may become sensitized to via the skin.23 The major allergen Der p 1 from Dermatophagoides pteronyssinus, a well-examined house dust mite allergen, is an enzyme that has a significant similarity to an enzyme from bromelain called papain. Papain might induce sensitization via destruction of the barrier function, resulting in dysfunctional skin.24,25 Its relevance for the skin barrier and allergy induction is explained by papain-usage as a component in cosmetics, pharmaceuticals, meat processing and in detergents (shampoo, washing powder etc.). Papain being similar to mite allergens might explain how these products can become a source for allergic diseases and their exacerbation.26,27 In a recent study papain was able to disrupt tight junctions and induce vasodilation as well as dryness in human keratinocytes.25 In mice, it increased inflammatory signaling cascades by recruiting neutrophils, mast cells and specific T-cells that function as promotors in antibody responses25 with enzyme activity present.28
Skin Care
Patients with atopic dermatitis, or genetically predisposed patients that have a higher risk for atopic eczema, are more likely to develop food allergies, asthma or rhinitis.29 Since not only mite allergens but any other allergen can enter through inflamed skin and cause allergies,30 the skin barrier must be properly protected in order to prevent chronic skin dysfunction through inflammation and therefore allergic comorbidities of atopic eczema.29 Skin emollients might be helpful here, however, in eczematous patients the composition and ingredients of emollients decide whether the symptoms are suppressed or triggered. In a four-week trial, patients with atopic dermatitis were treated with three different emollients and subsequently their emollient-treated skin was compared to non-treated areas of their skin.30 Conventional paraffin creams showed no protection against irritants and even reduced the skin’s natural moisturizing factor. Urea–glycerol creams did the opposite and reduced transepidermal water loss and dryness. Glycerol-containing moisturizers performed better than paraffin creams but could not protect the skin as good as urea-glycerol creams. In another interventional study, high-risk newborns with at least one first-degree relative having an allergic condition, were treated with emollients daily in their first year.31 However, compared to the control group there was no evidence of a reduced risk for eczema in these patients until the age of two. Additionally, a similar intervention with a daily one-year emollient treatment and a long-term follow-up at the age of three, four and five years of age reported no prevention of food allergy, asthma, hay fever or atopic dermatitis.32
There was also a significant association between the bathing frequency of three- and twelve-month-old babies and the elevation of transepidermal water loss, which is connected to skin barrier dysfunction. Daily bathing had the worst effect on transepidermal water loss compared to less frequent or very rare bathing, even after taking confounders into account like emollients, bath oils, wet wipes, filaggrin mutations, allergic parents, and similar. Still, the incidence of atopic dermatitis only increased with bathing frequencies among three-month-olds, but not twelve-month-olds.33
Nutrition
A fiber-rich diet influences the immunomodulation of the intestine by specific gene transcription and receptor activation, which helps maintain the gut homeostasis.12 Fibers are the raw material for production of short-chain fatty acids (SFA) by gut microbiota, such as acetate, butyrate and propionate, which can interact with G-protein-coupled receptors that induce anti-inflammatory signaling pathways maintaining a healthy gut. These pathways particularly influence the activation of regulatory T-cells, IgA antibodies and dendritic cells. Depending on the gut microbiome’s ability to digest fibers into short fatty acids, these metabolites can also induce epigenetic modulation in many cells and tissues.12,34 These findings suggest that the synergy of dietary food and commensal bacteria compositions have a significant effect on immunoregulatory systems. Accordingly, findings regarding dietary habits of African and European children highlight the relevance of the diet for the gut microbiome.35 Western European diet is rich in animal proteins, fat, starch and sugar, whereas rural African diet contains more fibers, vegetable-derived protein and plant polysaccharides. These dietary patterns and their effect on the microbiome may be the source of an increased prevalence of allergic conditions in Western countries. African children host gut bacteria like Prevotella and Xylanibacter that are completely absent among the fecal samples of European children. These bacteria are connected to very potent fiber digestion, indicating a co-development of the gut microbiota and dietary habits. In contrast to that, pathologic bacteria like Shigella and Escherichia were significantly higher in European children, but hardly verifiable in the African cohort.
Between children with and without food allergies by the age of two, early infant diet including breast-feeding, infant formula and the start of solid food introduction did not differ, however, a difference was found after solid food introduction: non-allergic children were introduced rather to fresh and home-made solid food, and less to processed dishes, grown-up food, or commercial baby food 28–42 weeks after birth.36 Children that developed food allergies were not fed as healthy (lower healthy infant diet dietary pattern score) after terminating breast feeding or formula. In summary, late infant dietary patterns that mostly include fruits, vegetables and home-made dishes are related to less food allergy development at the age of two years. This can again be explained by potential immunoregulatory functions of high levels of vitamin C, folate, oligosaccharides and beta-carotenes in fresh fruit and vegetables.
The farm effect
There are several potential factors that prevent allergy development in children living on a farm. Environmental conditions, gut microbes, animal metabolites and nutrition play a significant role in regulating immune reactions and protecting farm children from allergies and asthma. The so-called “farm effect” has already been observed in numerous studies.37 Consumption of raw milk, but also exposure to stables seem to shape relevant immune responses that prevent asthma, hay fever or atopic sensitization.38,39 Especially unprocessed farm milk is considered one of the main contributors to the protective effect. It consists of components that may act as health-promotors such as whey proteins, microRNA, polyunsaturated fatty acids, vitamins, and oligosaccharides.40 A recent study found higher levels of Treg cells like CD4+CD25+FOXP3+ T cells in farm-exposed children, underlining the allergy protective effect of raw milk.41
The consumption of raw milk may even independently, regardless of any other environmental influences, provide protection against allergic health issues in childhood, such as rhinoconjunctivitis and asthma.42 A recent study described epigenetic modifications of T-cells as a possible explanation for the development of allergy tolerance after raw milk consumptions in mice.43 In vitro, also effector cells of allergy (mast cells) incubated with raw milk as opposed to mast cells incubated with processed milk showed less immunoreactive responses.26 This led to the conclusion that raw milk directly interacts with mast cells during IgE-mediated immune responses. When the raw milk was separated into molecular fractions, it emerged that mainly components with a molecular weight over 35 kDa were able to oppress the mast cell reactivity.
The manipulation of (whey) proteins might be the main reason for the difference in immunogenicity of raw and processed milk. Bovine milk contains multiple proteins such as caseins, α-lactalbumin, β-lactoglobulin, serum albumin and many more.44 These proteins change during processing steps like heating, defatting or acidification, which can lead to their denaturation or aggregation changing their tolerogenicity and/or allergenicity.45,46 In an experimental animal study, the loss of the allergy-protective capacity of milk started at a heat exposure of 65°C, which went hand in hand with the loss or aggregation of whey proteins.46
One of the main whey proteins of bovine milk is β-lactoglobulin (BLG).47 It is present in a concentration of up to 3g/L milk, with a molecular mass of 18.4 kDa as monomer and approximately 36 kDa as dimer. Under physiological conditions it forms the dimeric state.48 Apart from bovine milk, BLG is also present in cattle stable dust, secreted by female as well as male cattle via urine, and in cattle farming households.49
BLG is a protein known for its dual immune-modulation characteristics: As a lipocalin48 it is a carrier protein for small hydrophobic ligands like iron-siderophores, vitamin A and D and fatty acids or hormones. In the farm dust, it was found bound to zinc.49 Depending on its loading state, it drives the immune reaction into either an allergic state (when unloaded) or prevents allergy induction (when loaded with binding partners), as shown in vitro and in vivo. Allergic mice that were treated intranasally with zinc-BLG containing dust, or fed retinol- or iron-catechin-loaded BLG (holoBLG) showed less antibody levels against BLG and even against the unrelated allergen Bet v 1 from birch.50 Also, their allergic reactions as well as their antigen presentation potential was lower compared to the control group that was fed empty BLG (apoBLG). In human individuals, BLG loaded with binding partners could even reduce an ongoing allergic response.49–53 Serum-IgE of milk-allergic children showed decreased binding to holoBLG, and mast cells stimulated with holoBLG decreased their allergen-specific degranulation.54 These findings show that supplementation with holoBLG is able to perform primary and secondary allergen-unspecific prevention of allergic diseases.
Summary
In summary, influence factors on development and proceeding of allergic diseases are manifold and compile endogenous as well as exogenous factors (Fig.1). Some factors might be rather complicated to integrate into daily life, like farm environment or pets in high numbers. Others can be manipulated easily, like prevention of skin barrier integrity via low frequency of bathing in young children and application of efficient emollient substances, an optimal diet starting already at very young age in life, and avoidance of active and passive smoking. Recently, in a One Health sense, also the environment comes into play, like contact with biodiverse natural areas (forests, microbe-rich soil), integrating greenness into private and office rooms (indoor green walls) as well as outdoors (gardening, enriched sandboxes for children). A major focus of politicians, city planners, health insurances, and also parents/caretakers should be put on these modifiable prevention factors to help reduce the allergy pandemic.
Literature:
1 Cantani A: Pediatric allergy, asthma and immunology. Springer Science & Business Media 2008 2 Litonjua AA et al.: Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med 1998; 158(1): 176-81 3 Stapleton M et al.: Smoking and asthma. J Am Board Fam Med 2011; 24(3): 313-22 4 Harju M et al.: Parental smoking and cessation during pregnancy and the risk of childhood asthma. BMC Public Health 2016; 16: 428 5 Vich Vila A et al.: Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun 2020; 11(1): 362 6 Jordakieva G et al.: Country-wide medical records infer increased allergy risk of gastric acid inhibition. Nat Commun 2019; 10(1): 3298 7 Brunner R et al.: Aluminium per se and in the anti-acid drug sucralfate promotes sensitization via the oral route. Allergy 2009; 64(6): 890-7 8 Brunner R et al.: The impact of aluminium in acid-suppressing drugs on the immune response of BALB/c mice. Clin Exp Allergy 2007; 37(10): 1566-73 9 Pali-Schöll I et al.: Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin Exp Allergy 2010; 40(7): 1091-8 10 Pali-Schöll I, Jensen-Jarolim E: Anti-acid medication as a risk factor for food allergy. Allergy 2011; 66(4): 469-77 11 Pali-Schöll I et al.: The effect of digestion and digestibility on allergenicity of food. Nutrients 2018; 10(9): 1129; 12 McKenzie C et al.: The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev 2017; 278(1): 277-95 13 Diesner SC et al.: A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin Immunol 2016; 173: 10-18 14 Untersmayr E et al.: Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 2003; 112(3): 616-23 15 Metsälä J et al.: Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy 2015; 45(1): 137-45 16 Stensballe LG et al.: Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr 2013; 162(4): 832-8.e3 17 Shu SA et al.: Microbiota and Food Allergy. Clin Rev Allergy Immunol 2019; 57(1): 83-97 18 Roslund MI et al.: Long-term biodiversity intervention shapes health-associated commensal microbiota among urban day-care children. Environ Int 2021; 157: 106811 19 Roslund MI et al.: A placebo-controlled double-blinded test of the biodiversity hypothesis of immune-mediated diseases: Environmental microbial diversity elicits changes in cytokines and increase in T regulatory cells in young children. Ecotoxicol Environ Saf 2022; 242: 113900 20 Soininen L et al.: Indoor green wall affects health-associated commensal skin microbiota and enhances immune regulation: a randomized trial among urban office workers. Sci Rep 2022; 12(1): 6518 21 Ojwang V et al.: Early exposure to cats, dogs and farm animals and the risk of childhood asthma and allergy. Pediatr Allergy Immunol 2020; 31(3): 265-72 22 Hesselmar B et al.: Pet-keeping in early life reduces the risk of allergy in a dose-dependent fashion. PLoS One 2018; 13(12): e0208472 23 Jackson AP et al.: Prevalence of house dust mites and dermatophagoides group 1 antigens collected from bedding, skin and hair coat of dogs in south-west England. Vet Dermatol 2005; 16(1): 32-8 24 Reithofer M, Jahn-Schmid B: Allergens with protease activity from house dust mites. Int J Mol Sci 2017; 18(7): 1368 25 Stremnitzer C et al.: Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57BL/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J Invest Dermatol 2015; 135(7): 1790-800 26 Abbring S et al.: Direct inhibition of the allergic effector response by raw cow‘s milk-an extensive in vitro assessment. Cells 2020; 9(5): 1258 27 Budnik LT et al.: Sensitising effects of genetically modified enzymes used in flavour, fragrance, detergence and pharmaceutical production: cross-sectional study. Occup Environ Med 2017; 74(1): 39-45 28 Ogasawara A et al.: Epicutaneous challenge with protease allergen requires its protease activity to recall T(H)2 and T(H)17/T(H)22 responses in mice pre-sensitized via distant skin. J Immunotoxicol 2021; 18(1): 118-26 29 Maintz L et al.: From skin barrier dysfunction to systemic impact of atopic dermatitis: implications for a precision approach in dermocosmetics and medicine. J Pers Med 2022; 12(6): 893 30 Danby SG et al.: Different types of emollient cream exhibit diverse physiological effects on the skin barrier in adults with atopic dermatitis. Clin Exp Dermatol 2022; 47(6): 1154-64 31 Chalmers JR et al.: Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020; 395(10228): 962-72 32 Bradshaw LE et al.: Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy 2022 33 Marrs T et al.: Bathing frequency is associated with skin barrier dysfunction and atopic dermatitis at three months of age. J Allergy Clin Immunol Pract 2020; 8(8): 2820-22 34 Trompette A et al.: Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014; 20(2): 159-66 35 De Filippo C et al.: Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010; 107(33): 14691-6 36 Grimshaw KE et al.: Diet and food allergy development during infancy: birth cohort study findings using prospective food diary data. J Allergy Clin Immunol 2014; 133(2): 511-9 37 Frei R et al.: Environmental influences on childhood allergies and asthma - the farm effect. Pediatr Allergy Immunol 2022; 33(6): e13807 38 Riedler J et al.: Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001; 358(9288): 1129-33 39 Mayerhofer H et al.: The extended farm effect: The milk protein β-lactoglobulin in stable dust protects against allergies. Allergol Select 2022; 6: 111-7 40 Brick T et al.: The beneficial effect of farm milk consumption on asthma, allergies, and infections: From meta-analysis of evidence to clinical trial. J Allergy Clin Immunol Pract 2020; 8(3): 878-89.e3 41 Lluis A et al.: Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol 2014; 133(2): 551-9 42 Waser M et al.: Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy 2007; 37(5): 661-70 43 Abbring S et al.: Raw cow‘s milk reduces allergic symptoms in a murine model for food allergy-a potential role for epigenetic modifications. Nutrients 2019; 11(8): 1721 44 Crittenden RG, Bennett LE: Cow‘s milk allergy: a complex disorder. J Am Coll Nutr 2005; 24(6 Suppl): 582s-591s 45 Jensen SA et al.: Diagnosis and rationale for action against cow‘s milk allergy (DRACMA) Guidelines update - III - Cow‘s milk allergens and mechanisms triggering immune activation. World Allergy Organ J 2022; 15(9): 100668 46 Abbring S et al.: Loss of allergy-protective capacity of raw cow‘s milk after heat treatment coincides with loss of immunologically active whey proteins. Food Funct 2020; 11(6): 4982-93 47 Wong DW et al.: Structures and functionalities of milk proteins. Crit Rev Food Sci Nutr 1996; 36(8): 807-44 48 Sawyer L, Kontopidis G: The core lipocalin, bovine beta-lactoglobulin. Biochim Biophys Acta 2000; 1482(1-2): 136-48 49 Pali-Schöll I et al.: Secretory protein beta-lactoglobulin in cattle stable dust may contribute to the allergy-protective farm effect. Clin Transl Allergy 2022; 12(2): e12125 50 Afify SM et al.: Bovine holo-beta-lactoglobulin cross-protects against pollen allergies in an innate manner in BALB/c mice: potential model for the farm effect. Front Immunol 2021; 12: 611474 51 Bartosik T et al.: Ameliorating atopy by compensating micronutritional deficiencies in immune cells: a double-blind placebo-controlled pilot study. J Allergy Clin Immunol Pract 2022; 10(7): 1889-902.e9 52 Roth-Walter F et al.: Cow‘s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J Allergy Clin Immunol 2021; 147(1): 321-34.e4 53 Bergmann K-C RJ et al.: Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo Journal International 2022; 31: 161-71 54 Afify SM et al.: Micronutritional supplementation with a holoBLG-based FSMP (food for special medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: Imitating the protective farm effect. Clin Exp Allergy 2022; 52(3): 426-41
Das könnte Sie auch interessieren:
Biologika-Therapie: Pipeline und Klinik
Biologika bieten die Option, in die hinter der atopischen Dermatitis stehenden Immunprozesse gezielt einzugreifen. Dieser Weg wurde zuerst mit dem Anti-IL-4/IL-13-Antikörper Dupilumab ...
Zentrale Aspekte bei atopischer Dermatitis
Über die medikamentöse Therapie hinaus bleiben die Berücksichtigung von Umweltfaktoren, die Sicherstellung der Adhärenz und die Anpassung der Therapie an Begleiterkrankungen oder ...
Neuer Wirkstoff bei Acne vulgaris
Acne vulgaris führt vor allem während der Pubertät zu Leidensdruck bei Betroffenen. Zur Behandlung stehen je nach Ausprägung verschiedene Therapeutika zur Verfügung, kürzlich wurde der ...